| INNOVATING FOR PATIENTS Nasdaq TRVN I January 2023 |
| Forward - Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts regarding Trevena, Inc. (t he “Company” or “we”), they are forward - looking statements reflecting management’s current beliefs and expectations. Forward - looking statements are subject to known and unknown risks, unc ertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward - looking statements by terminology such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “objective,” “predict,” “pr oject,” “suggest,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “ongoing,” or the negative of these terms or similar expressions. Forward - looking statements contained in this prese ntation include, but are not limited to, ( i ) statements regarding the timing of anticipated clinical trials for our product candidates; (ii) the timing of receipt of clinical data for our pro duc t candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the size of patient populations targeted by our product candidates and ma rke t adoption of our potential drugs by physicians and patients; (v) the timing or likelihood of regulatory filings and approvals; and (vi) our cash needs. Actual results may differ materially from those indicated by such forward - looking statements as a result of various important f actors, including: the commercialization of any approved drug product, the status, timing, costs, results and interpretation of our clinical trials or any future trials of any of our inve sti gational drug candidates; the uncertainties inherent in conducting clinical trials; expectations for regulatory interactions, submissions and approvals, including our assessment of the discussions with th e FDA or other regulatory agencies about any and all of our programs; uncertainties related to the commercialization of OLINVYK; available funding; uncertainties related to our intellec tua l property; uncertainties related to the ongoing COVID - 19 pandemic, other matters that could affect the availability or commercial potential of our therapeutic candidates; and other f act ors discussed in the Risk Factors set forth in our Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q filed with the Securities and Exchange Commission (SEC) and in other filings we make with the SEC from time to time. In addition, the forward - looking statements included in this presentation represent our views only as of the date hereof. We anticipate that subs equent events and developments may cause our views to change. However, while we may elect to update these forward - looking statements at some point in the future, we specifically disc laim any obligation to do so, except as may be required by law. 2 |
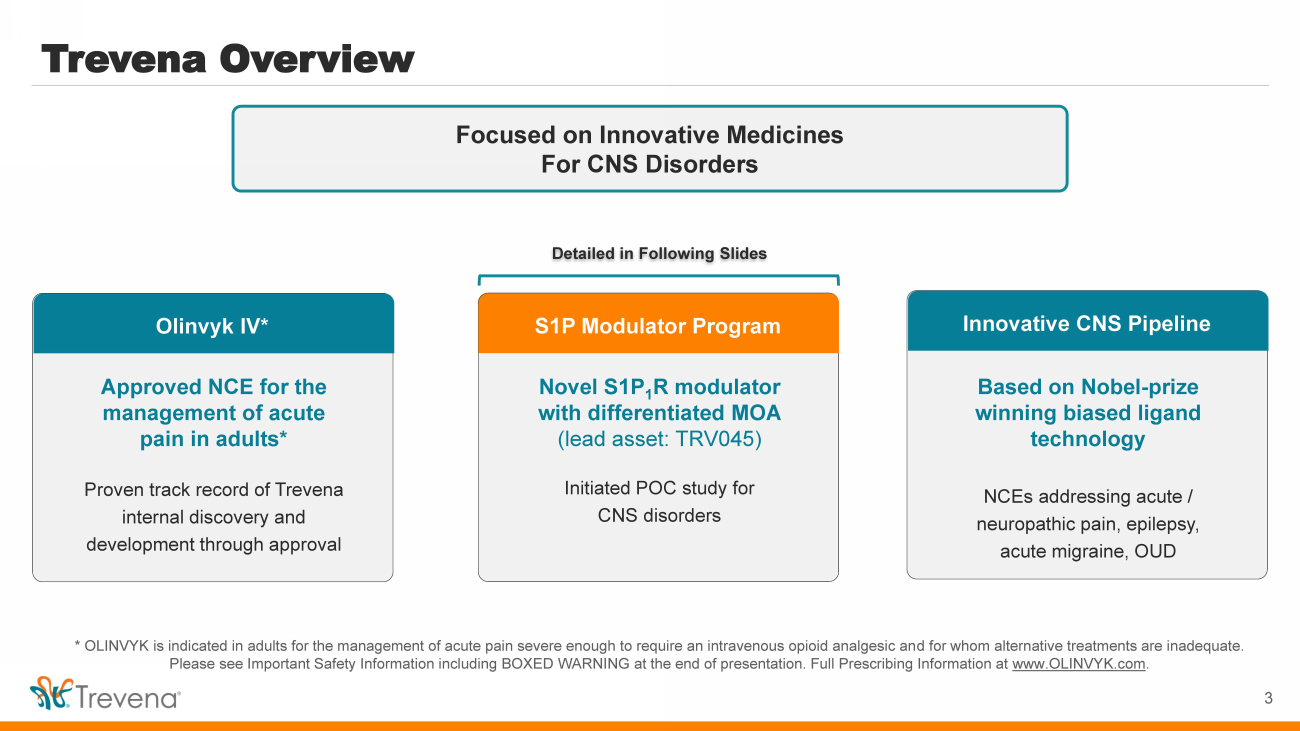
| Trevena Overview 3 Focused on Innovative Medicines For CNS Disorders Olinvyk IV* Approved NCE for the management of acute pain in adults* Proven track record of Trevena internal discovery and development through approval S1P Modulator Program Novel S1P 1 R modulator with differentiated MOA (lead asset: TRV045) Initiated POC study for CNS disorders Innovative CNS Pipeline Based on Nobel - prize winning biased ligand technology NCEs addressing acute / neuropathic pain, epilepsy, acute migraine, OUD Detailed in Following Slides * OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic an d f or whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com .. |
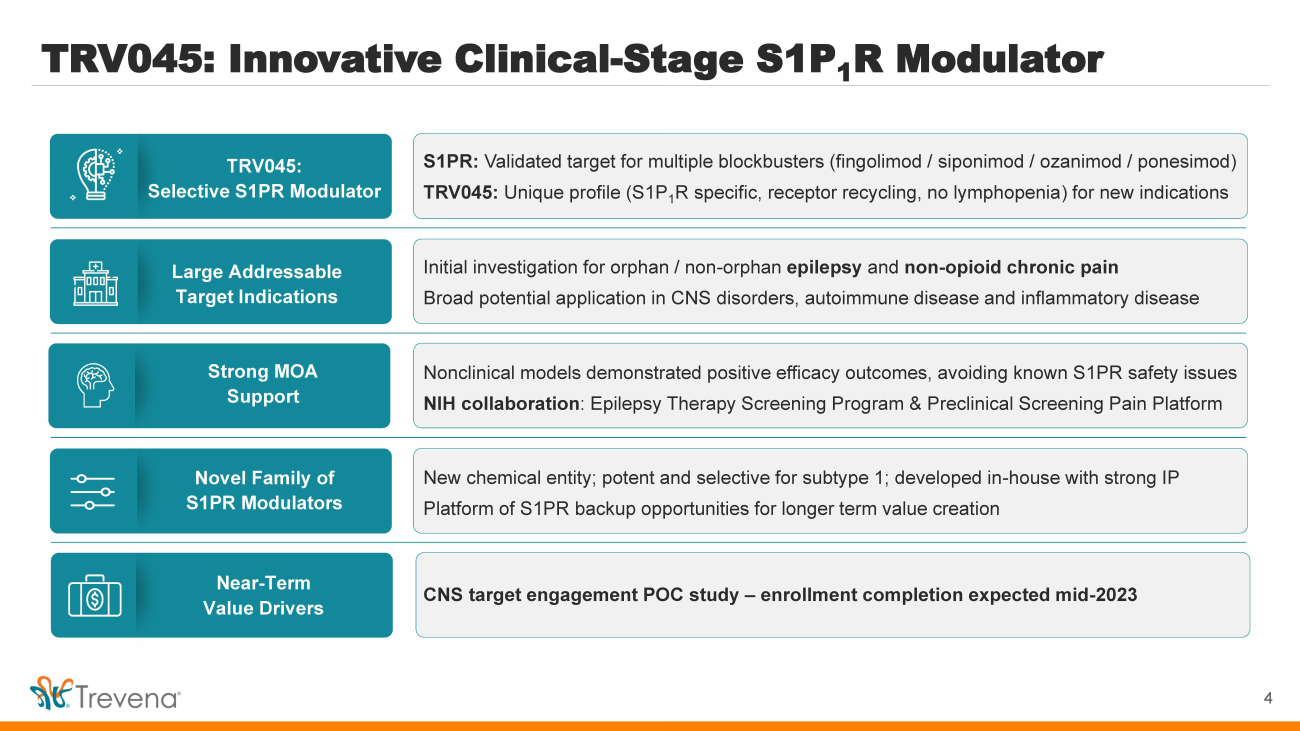
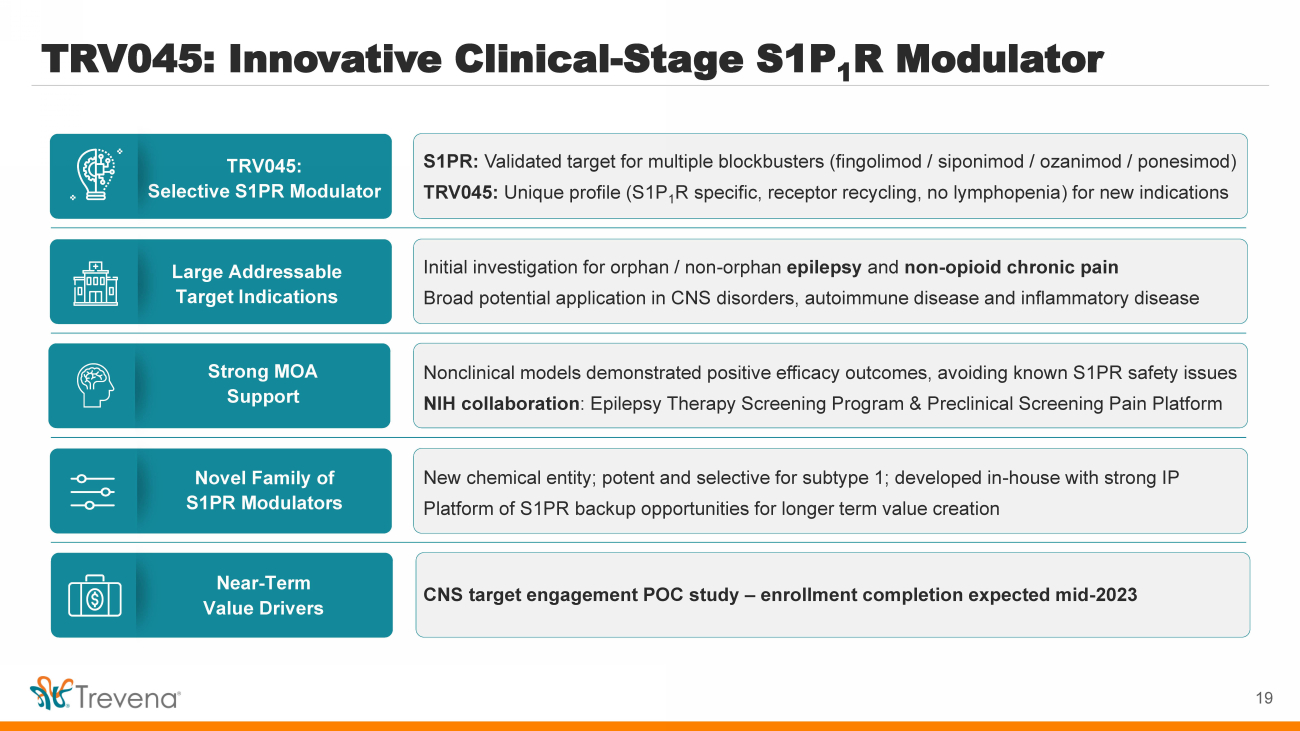
| TRV045: Innovative Clinical - Stage S1P 1 R Modulator TRV045: Selective S1PR Modulator Large Addressable Target Indications Novel Family of S1PR Modulators Strong MOA Support Near - Term Value Drivers S1PR: Validated target for multiple blockbusters (fingolimod / siponimod / ozanimod / ponesimod ) TRV045: Unique profile (S1P 1 R specific, receptor recycling, no lymphopenia ) for new indications Initial investigation for orphan / non - orphan epilepsy and non - opioid chronic pain Broad potential application in CNS disorders, autoimmune disease and inflammatory disease New chemical entity; potent and selective for subtype 1; developed in - house with strong IP Platform of S1PR backup opportunities for longer term value creation Nonclinical models demonstrated positive efficacy outcomes, avoiding known S1PR safety issues NIH collaboration : Epilepsy Therapy Screening Program & Preclinical Screening Pain Platform CNS target engagement POC study – enrollment completion expected mid - 2023 4 |
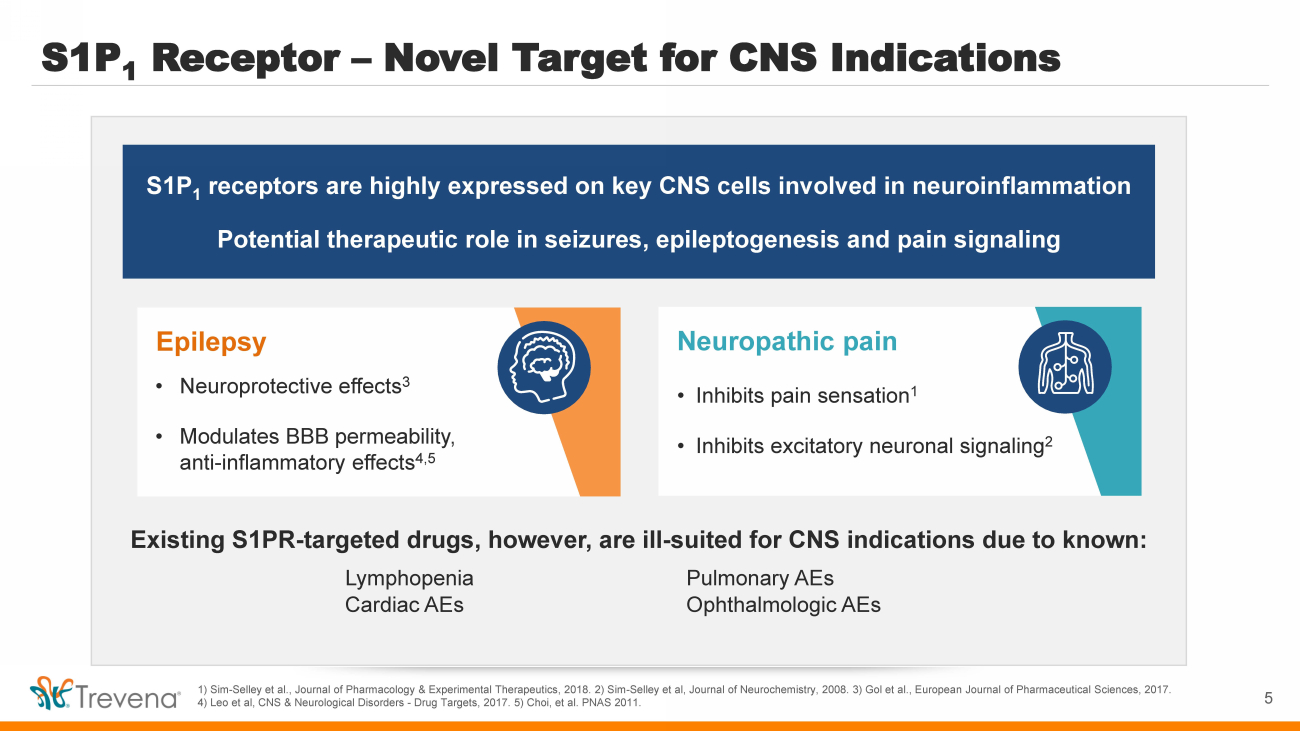
| S1P 1 Receptor – Novel Target for CNS Indications 1) Sim - Selley et al., Journal of Pharmacology & Experimental Therapeutics, 2018. 2) Sim - Selley et al, Journal of Neurochemistry, 2008. 3) Gol et al., European Journal of Pharmaceutical Sciences, 2017. 4) Leo et al, CNS & Neurological Disorders - Drug Targets, 2017. 5) Choi, et al. PNAS 2011. S1P 1 receptors are highly expressed on key CNS cells involved in neuroinflammation Potential therapeutic role in seizures, epileptogenesis and pain signaling Neuropathic pain • Inhibits pain sensation 1 • Inhibits excitatory neuronal signaling 2 Existing S1PR - targeted drugs, however, are ill - suited for CNS indications due to known: Lymphopenia Pulmonary AEs Cardiac AEs Ophthalmologic AEs Epilepsy • Neuroprotective effects 3 • Modulates BBB permeability, anti - inflammatory effects 4,5 5 |
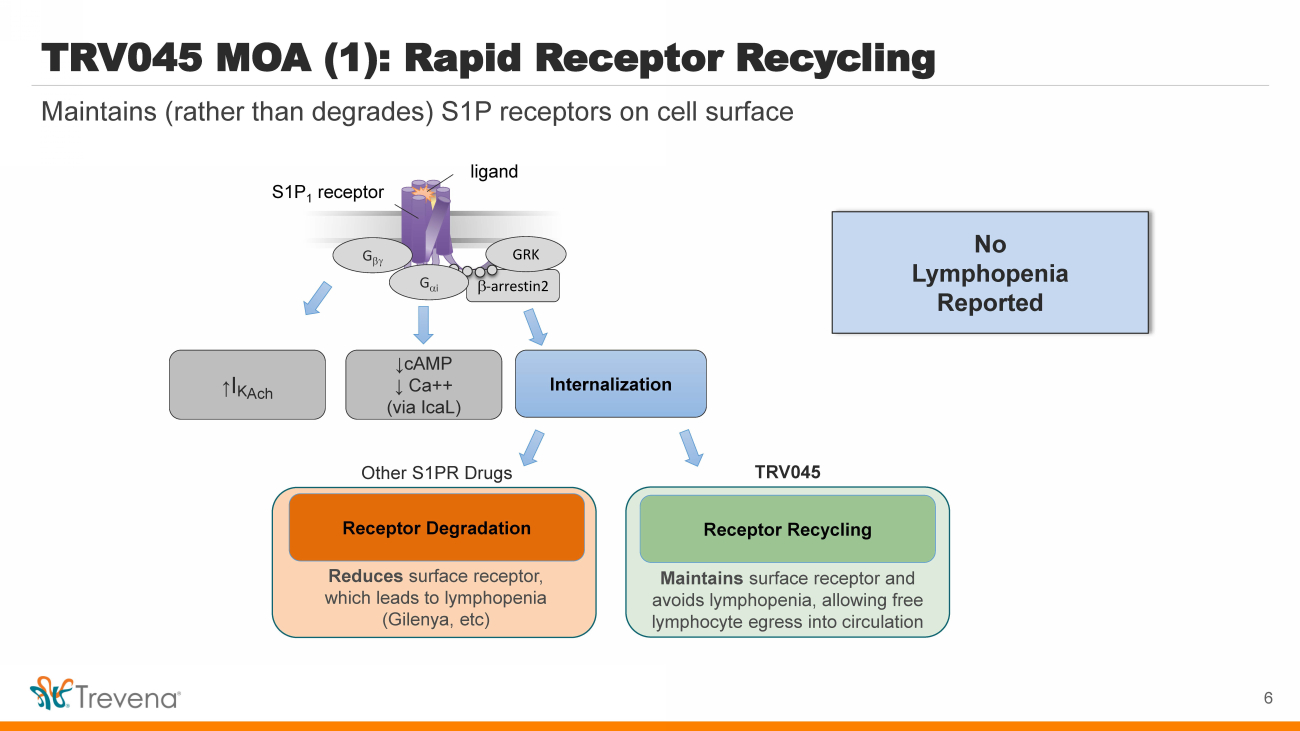
| TRV045 MOA (1): Rapid Receptor Recycling b - arrestin2 S1P 1 receptor G b GRK Internalization ↑ I K Ach ligand G a i ↓cAMP ↓ Ca++ (via IcaL ) Reduces surface receptor, which leads to lymphopenia ( Gilenya , etc ) Receptor R ecycling Maintains surface receptor and avoids lymphopenia, allowing free lymphocyte egress into circulation Receptor Degradation Other S1PR Drugs TRV045 6 Maintains (rather than degrades) S1P receptors on cell surface No Lymphopenia Reported |
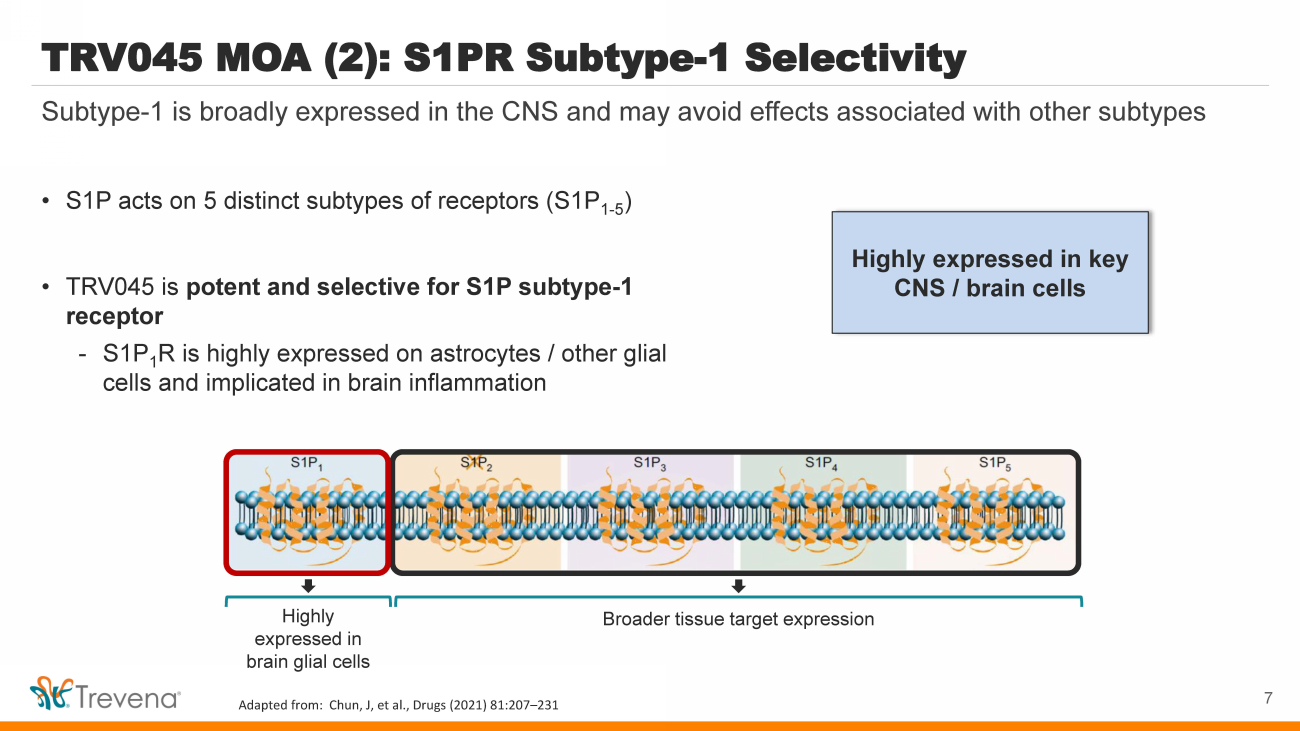
| Highly expressed in key CNS / brain cells TRV045 MOA (2): S1PR Subtype - 1 Selectivity • S1P acts on 5 distinct subtypes of receptors (S1P 1 - 5 ) • TRV045 is potent and selective for S1P subtype - 1 receptor - S1P 1 R is highly expressed on astrocytes / other glial cells and implicated in brain inflammation 7 Subtype - 1 is broadly expressed in the CNS and may avoid effects associated with other subtypes Highly expressed in brain glial cells Broader tissue target expression Adapted from: Chun, J, et al., Drugs (2021) 81:207 – 231 |
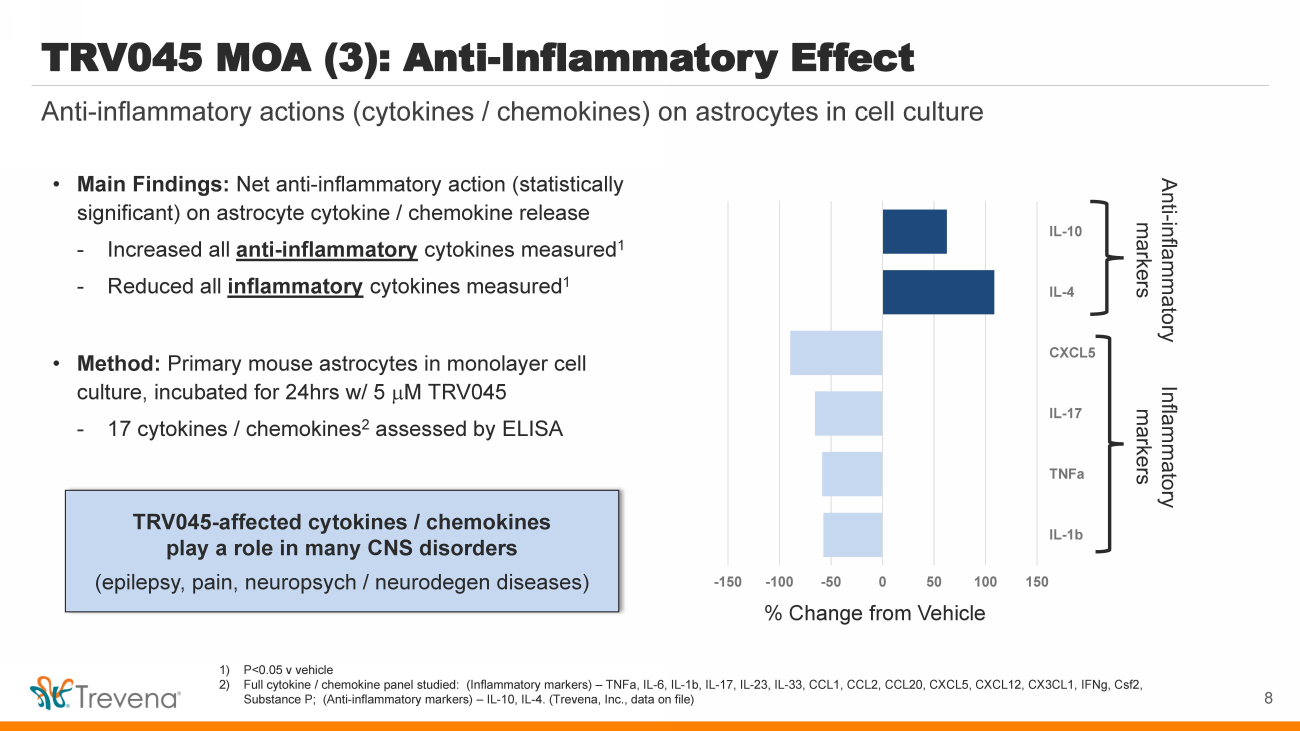
| • Main Findings: Net anti - inflammatory action (statistically significant) on astrocyte cytokine / chemokine release - Increased all anti - inflammatory cytokines measured 1 - Reduced all inflammatory cytokines measured 1 • Method: Primary mouse astrocytes in monolayer cell culture, incubated for 24hrs w/ 5 m M TRV045 - 17 cytokines / chemokines 2 assessed by ELISA TRV045 - affected cytokines / chemokines play a role in many CNS disorders (epilepsy, pain, neuropsych / neurodegen diseases) TRV045 MOA (3): Anti - Inflammatory Effect Anti - inflammatory actions (cytokines / chemokines) on astrocytes in cell culture 1) P<0.05 v vehicle 2) Full cytokine / chemokine panel studied: (Inflammatory markers) – TNFa , IL - 6, IL - 1b, IL - 17, IL - 23, IL - 33, CCL1, CCL2, CCL20, CXCL5, CXCL12, CX3CL1, IFNg , Csf2, Substance P; (Anti - inflammatory markers) – IL - 10, IL - 4. (Trevena, Inc., data on file) 8 -150 -100 -50 0 50 100 150 IL-1b TNFa IL-17 CXCL5 IL-4 IL-10 % Change from Vehicle Anti - inflammatory markers Inflammatory markers |
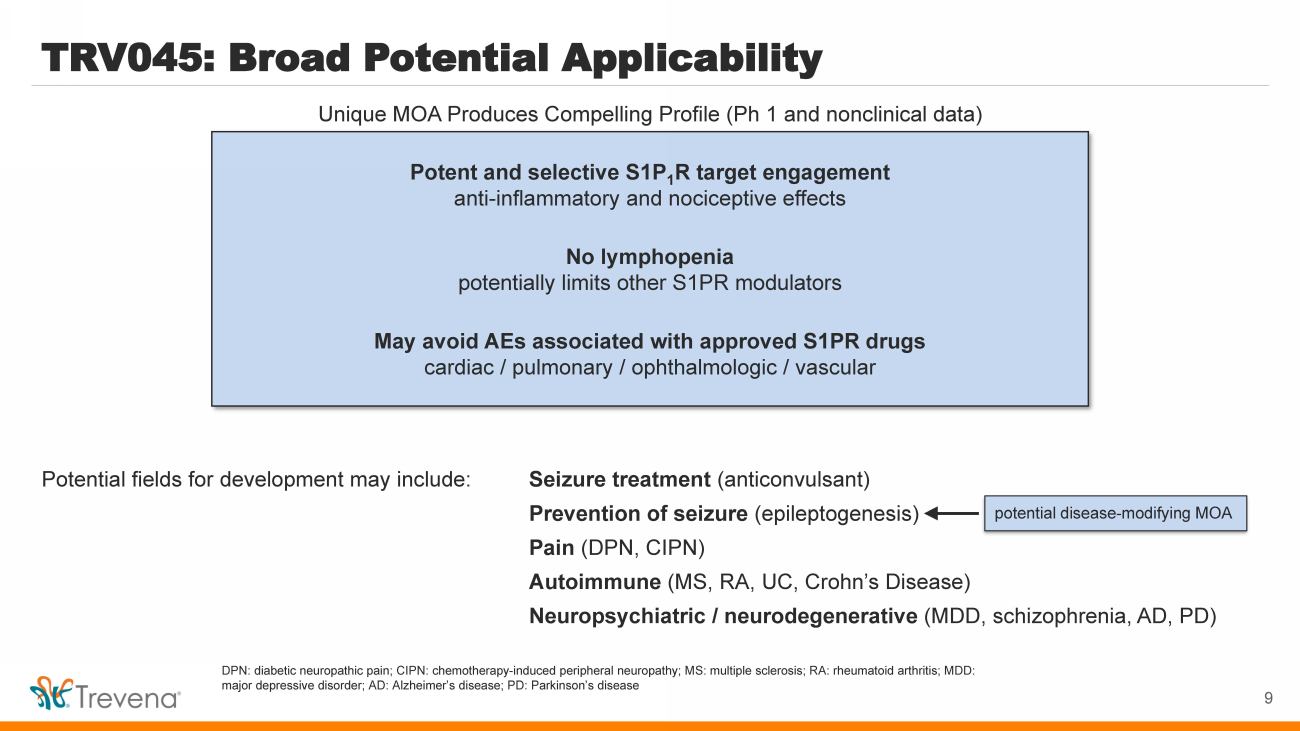
| TRV045: Broad Potential Applicability Potential fields for development may include: Seizure treatment (anticonvulsant) Prevention of seizure ( epileptogenesis ) Pain (DPN, CIPN) Autoimmune (MS, RA, UC, Crohn’s Disease) Neuropsychiatric / neurodegenerative (MDD, schizophrenia, AD, PD) Potent and selective S1P 1 R target engagement anti - inflammatory and nociceptive effects No lymphopenia potentially limits other S1PR modulators May avoid AEs associated with approved S1PR drugs cardiac / pulmonary / ophthalmologic / vascular 9 Unique MOA Produces Compelling Profile (Ph 1 and nonclinical data) p otential disease - modifying MOA DPN: diabetic neuropathic pain; CIPN: chemotherapy - induced peripheral neuropathy; MS: multiple sclerosis; RA: rheumatoid arthrit is; MDD: major depressive disorder; AD: Alzheimer’s disease; PD: Parkinson’s disease |
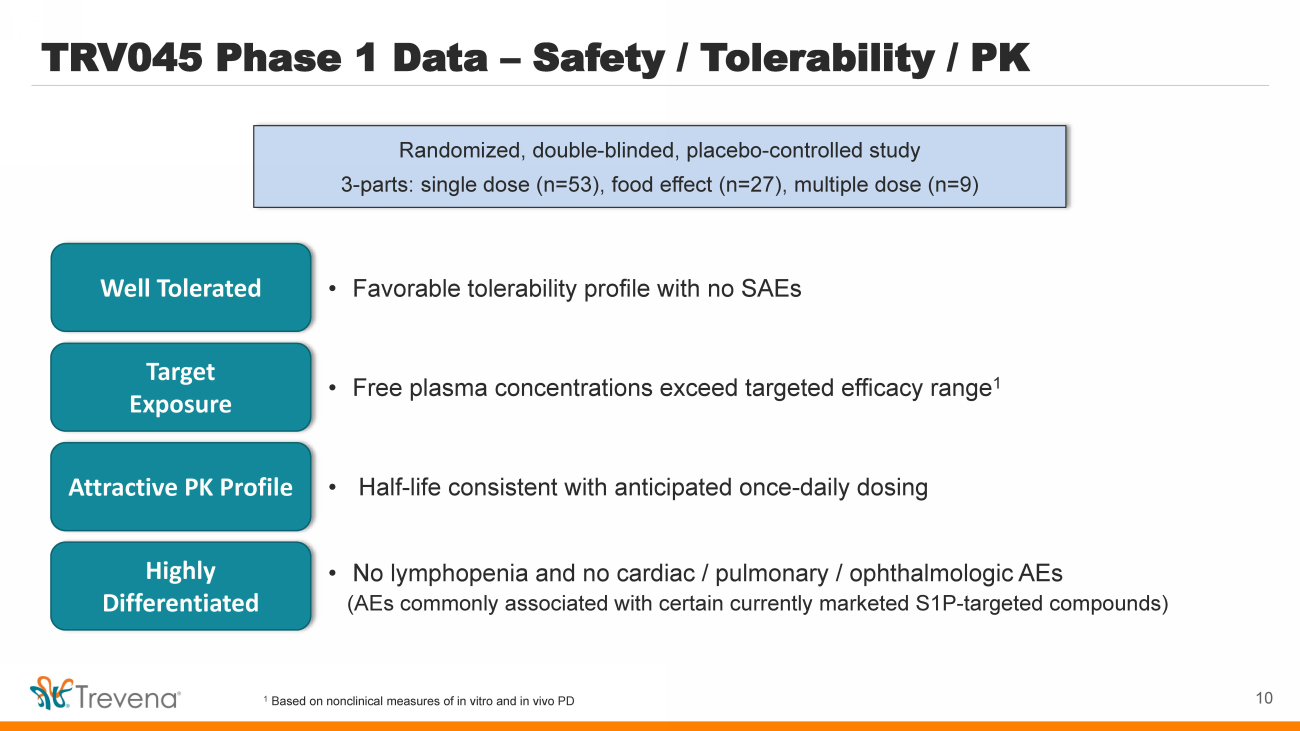
| TRV045 Phase 1 Data – Safety / Tolerability / PK Randomized, double - blinded, placebo - controlled study 3 - parts: single dose (n=53), food effect (n=27), multiple dose (n=9) • Favorable tolerability profile with no SAEs Well Tolerated Target Exposure Attractive PK Profile • Free plasma concentrations exceed targeted efficacy range 1 • Half - life consistent with anticipated once - daily dosing Highly Differentiated • No lymphopenia and no cardiac / pulmonary / ophthalmologic AEs (AEs commonly associated with certain currently marketed S1P - targeted compounds) 1 Based on nonclinical measures of in vitro and in vivo PD 10 |
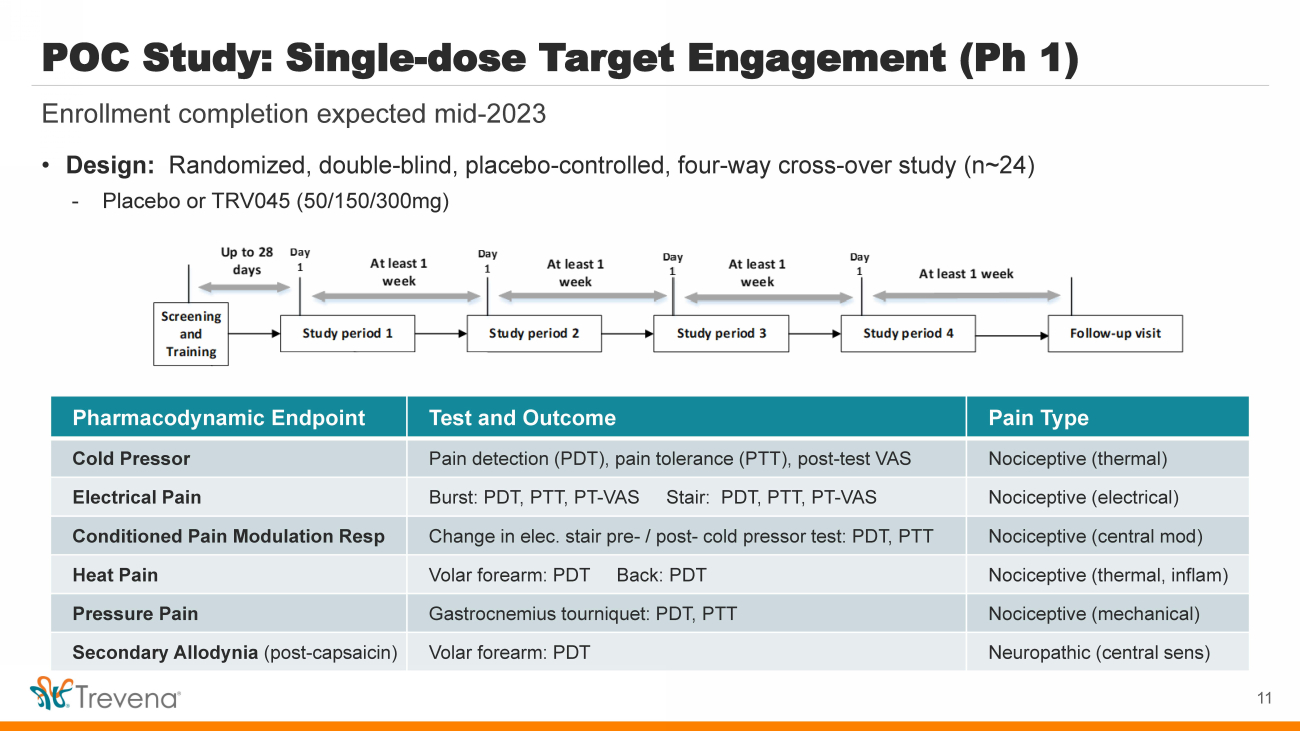
| POC Study: Single - dose Target Engagement (Ph 1) Enrollment completion expected mid - 2023 Pharmacodynamic Endpoint Test and Outcome Pain Type Cold Pressor Pain detection (PDT), pain tolerance (PTT), post - test VAS Nociceptive (thermal) Electrical Pain Burst: PDT, PTT, PT - VAS Stair: PDT, PTT, PT - VAS Nociceptive (electrical) Conditioned Pain Modulation Resp Change in elec. stair pre - / post - cold pressor test: PDT, PTT Nociceptive (central mod) Heat Pain Volar forearm: PDT Back: PDT Nociceptive (thermal, inflam ) Pressure Pain Gastrocnemius tourniquet: PDT, PTT Nociceptive (mechanical) Secondary Allodynia (post - capsaicin) Volar forearm: PDT Neuropathic (central sens ) 11 • Design: Randomized, double - blind, placebo - controlled, four - way cross - over study (n~24) - Placebo or TRV045 (50/150/300mg) |
| TRV045 Epilepsy and Related Indications |
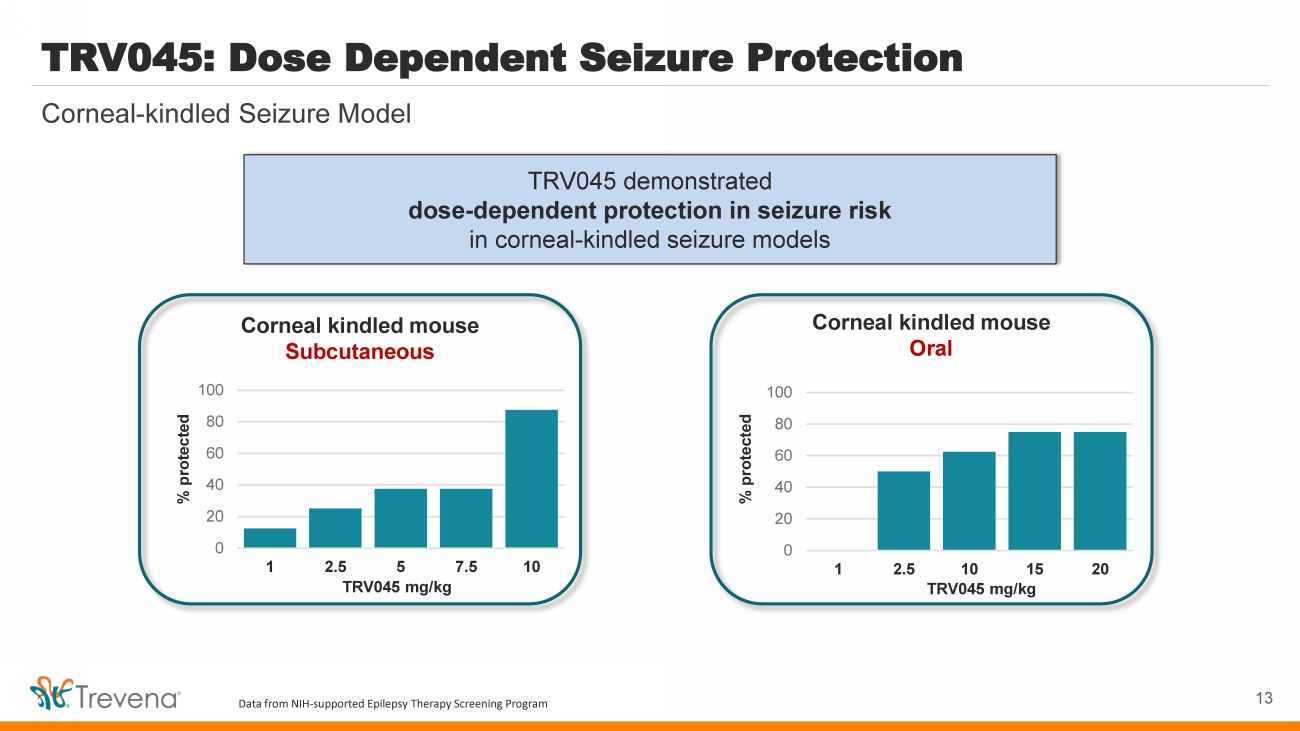
| TRV045 demonstrated dose - dependent protection in seizure risk in corneal - kindled seizure models Corneal - kindled Seizure Model TRV045: Dose Dependent Seizure Protection 13 Corneal kindled mouse Subcutaneous 0 20 40 60 80 100 1 2.5 5 7.5 10 % protected TRV045 mg/kg Corneal kindled mouse Oral 0 20 40 60 80 100 1 2.5 10 15 20 % protected TRV045 mg/kg Data from NIH - supported Epilepsy Therapy Screening Program |
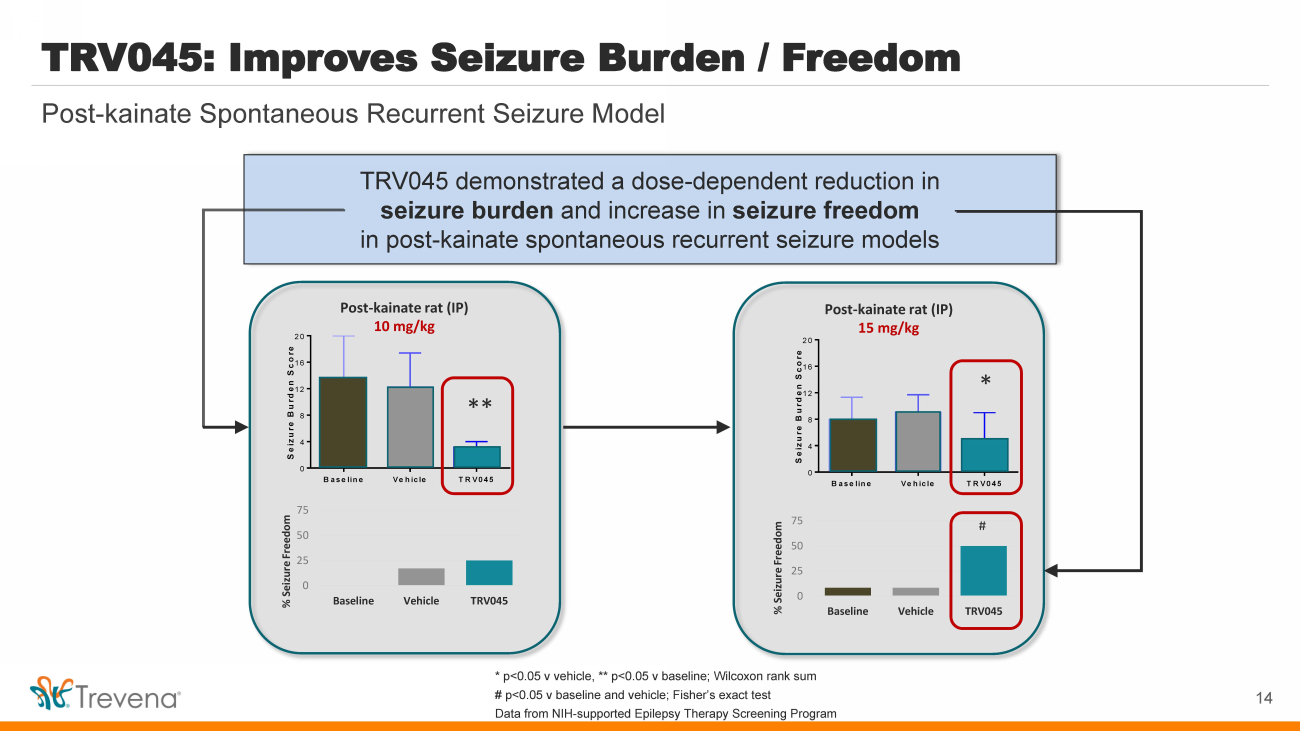
| Baseline Vehicle TRV045 0 4 8 12 16 20 Seizure Burden Score * Post - kainate rat (IP) 15 mg/kg * # 0 25 50 75 Baseline Vehicle TRV045 % Seizure Freedom Baseline Vehicle TRV045 0 4 8 12 16 20 Seizure Burden Score * Post - kainate rat (IP) 10 mg/kg ** 0 25 50 75 Baseline Vehicle TRV045 % Seizure Freedom TRV045 demonstrated a dose - dependent reduction in seizure burden and increase in seizure freedom in post - kainate spontaneous recurrent seizure models Post - kainate Spontaneous Recurrent Seizure Model TRV045: Improves Seizure Burden / Freedom 14 * p<0.05 v vehicle, ** p<0.05 v baseline; Wilcoxon rank sum # p<0.05 v baseline and vehicle; Fisher’s exact test Data from NIH - supported Epilepsy Therapy Screening Program |
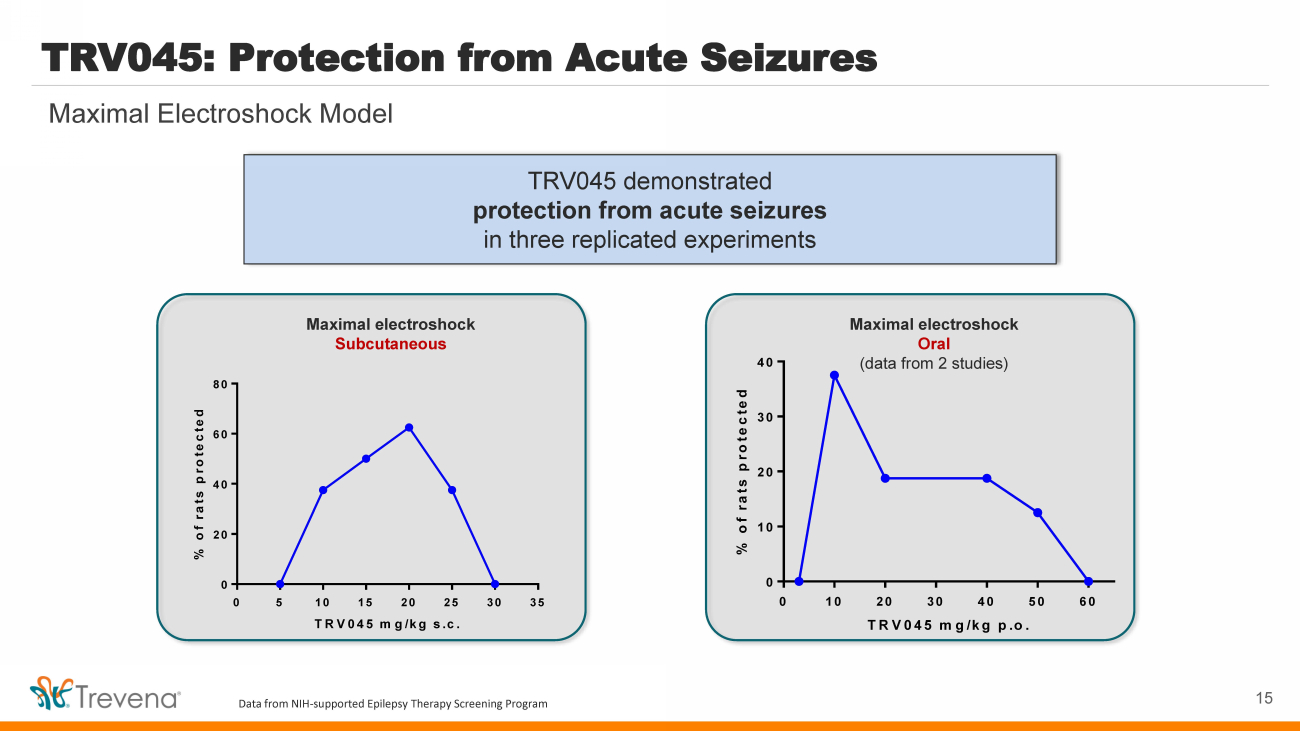
| TRV045 demonstrated protection from acute seizures in three replicated experiments TRV045: Protection from Acute Seizures 15 Maximal Electroshock Model 0 5 10 15 20 25 30 35 0 20 40 60 80 TRV045 mg/kg s.c. % of rats protected Maximal electroshock Subcutaneous 0 10 20 30 40 50 60 0 10 20 30 40 TRV045 mg/kg p.o. % of rats protected Maximal electroshock Oral (data from 2 studies) Data from NIH - supported Epilepsy Therapy Screening Program |
| TRV045 Non - opioid Pain Indications |
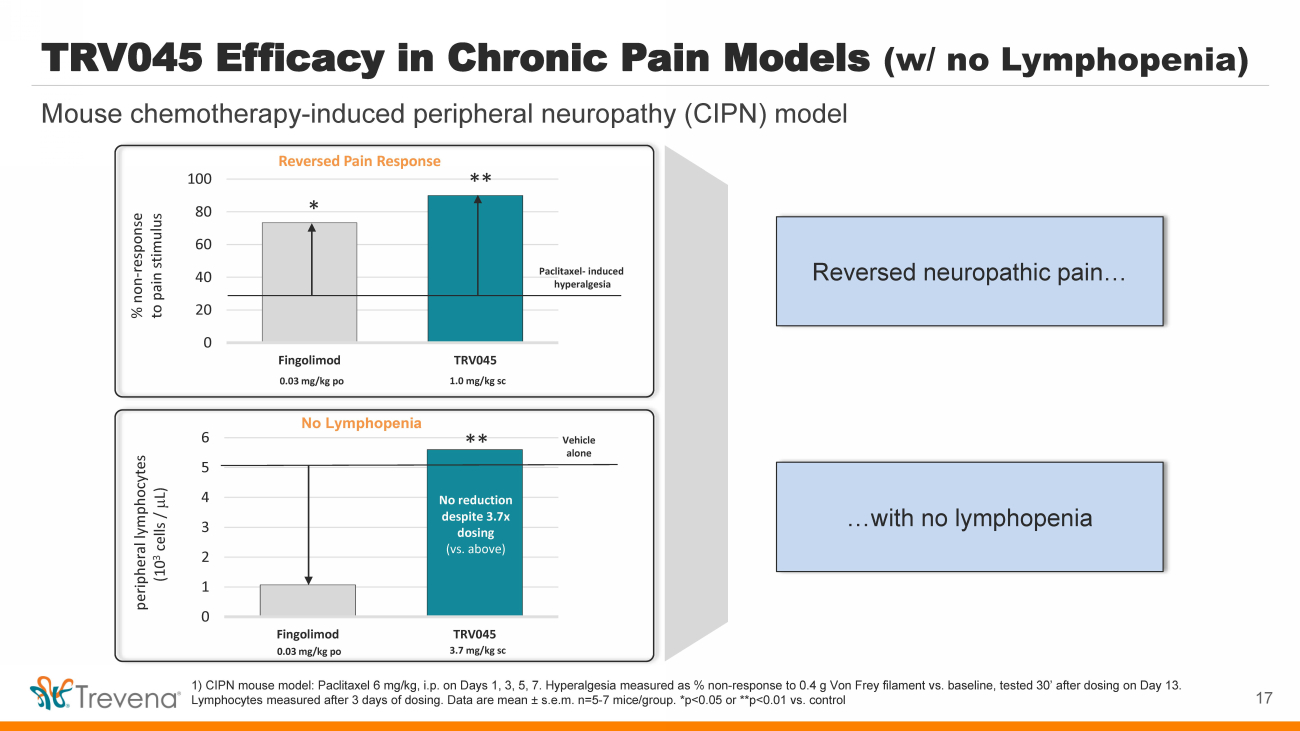
| 0 1 2 3 4 5 6 Fingolimod TRV045 No Lymphopenia TRV045 Efficacy in Chronic Pain Models (w/ no Lymphopenia) 1) CIPN mouse model: Paclitaxel 6 mg/kg, i.p. on Days 1, 3, 5, 7. Hyperalgesia measured as % non - response to 0.4 g Von Frey filament vs. baseline, tested 30’ after dosing on Day 13. Lymphocytes measured after 3 days of dosing. Data are mean ± s.e.m. n=5 - 7 mice/group. *p<0.05 or **p<0.01 vs. control peripheral lymphocytes (10 3 cells / m L) 0.03 mg/kg po 3.7 mg/kg sc Vehicle alone No reduction despite 3.7x dosing (vs. above) ** Reversed Pain Response 0 20 40 60 80 100 Fingolimod TRV045 % non - response to pain stimulus 0.03 mg/kg po 1.0 mg/kg sc Paclitaxel - induced hyperalgesia * ** 17 Mouse chemotherapy - induced peripheral neuropathy (CIPN) model Reversed neuropathic pain… …with no lymphopenia |
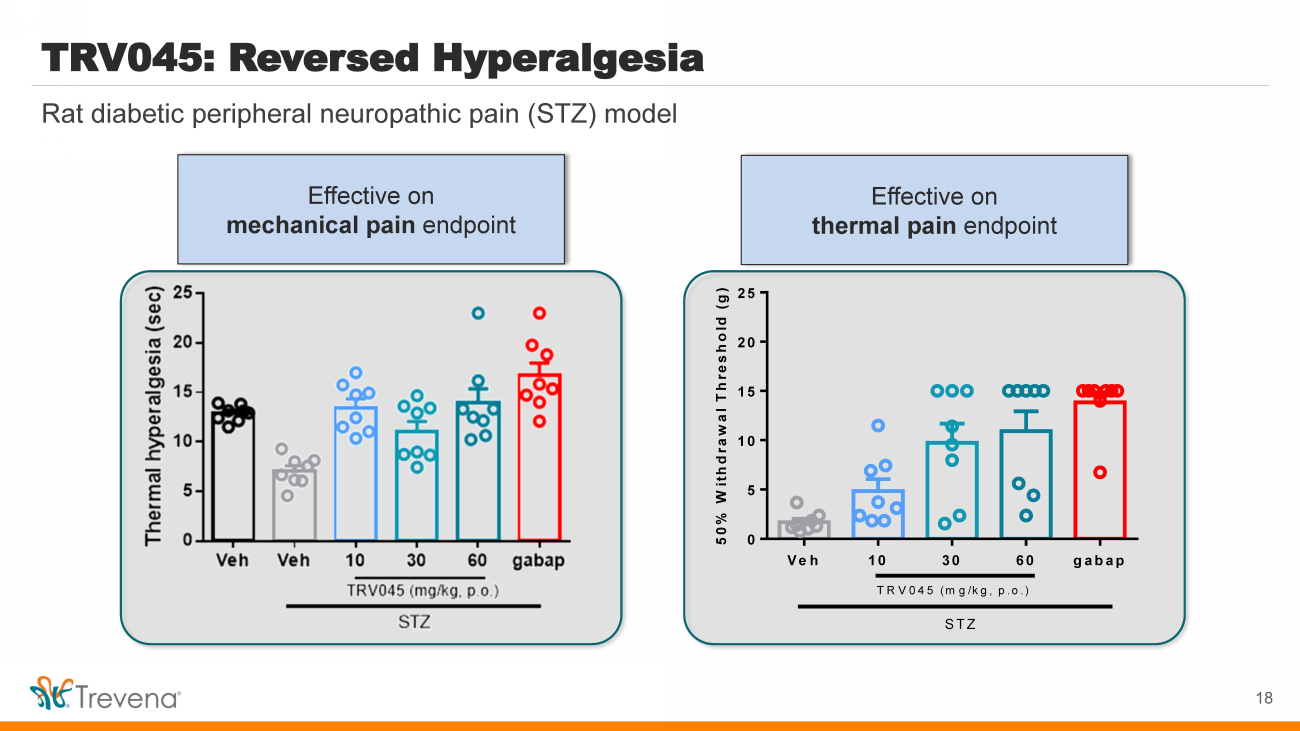
| Rat diabetic peripheral neuropathic pain (STZ) model TRV045: Reversed Hyperalgesia 18 Veh 10 30 60 gabap 0 5 10 15 20 25 Mehcanical allodynia 50% Withdrawal Threshold (g) STZ TRV045 (mg/kg, p.o.) Mechanical allodynia Thermal hyperalgesia Effective on thermal pain endpoint Effective on mechanical pain endpoint |
| TRV045: Innovative Clinical - Stage S1P 1 R Modulator TRV045: Selective S1PR Modulator Large Addressable Target Indications Novel Family of S1PR Modulators Strong MOA Support Near - Term Value Drivers S1PR: Validated target for multiple blockbusters (fingolimod / siponimod / ozanimod / ponesimod ) TRV045: Unique profile (S1P 1 R specific, receptor recycling, no lymphopenia ) for new indications Initial investigation for orphan / non - orphan epilepsy and non - opioid chronic pain Broad potential application in CNS disorders, autoimmune disease and inflammatory disease New chemical entity; potent and selective for subtype 1; developed in - house with strong IP Platform of S1PR backup opportunities for longer term value creation Nonclinical models demonstrated positive efficacy outcomes, avoiding known S1PR safety issues NIH collaboration : Epilepsy Therapy Screening Program & Preclinical Screening Pain Platform CNS target engagement POC study – enrollment completion expected mid - 2023 19 |
| 20 JL: can will create a transition slide? IMPORTANT OLINVYK SAFETY INFORMATION |
| WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE - THREATENING RESPIRATORY DEPRESSION; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CENTRAL NERVOUS SYSTEM (CNS) DEPRESSANTS Addiction, Abuse, and Misuse OLINVYK exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk before prescribing OLINVYK, and monitor all patients regularly for the development of behaviors or conditions. Life - Threatening Respiratory Depression Serious, life - threatening, or fatal respiratory depression may occur with use of OLINVYK. Monitor for respiratory depression, especially during initiation of OLINVYK or following a dose increase. Neonatal Opioid Withdrawal Syndrome Prolonged use of OLINVYK during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life - threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. Risk From Concomitant Use With Benzodiazepines or Other CNS Depressants Concomitant use of opioids with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation. Limitations of Use Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, reserve OLINVYK for use in patients for whom alternative treatment options [e.g., non - opioid analgesics or opioid combination products]: • Have not been tolerated, or are not expected to be tolerated • Have not provided adequate analgesia, or are not expected to provide adequate analgesia. The cumulative total daily dose should not exceed 27 mg, as total daily doses greater than 27 mg may increase the risk for QTc interval prolongation. CONTRAINDICATIONS OLINVYK is contraindicated in patients with: • Significant respiratory depression • Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment • Known or suspected gastrointestinal obstruction, including paralytic ileus • Known hypersensitivity to oliceridine (e.g., anaphylaxis) WARNINGS AND PRECAUTIONS • OLINVYK contains oliceridine, a Schedule II controlled substance, that exposes users to the risks of addiction, abuse, and misuse. Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed OLINVYK. Assess risk, counsel, and monitor all patients receiving opioids. • Serious, life - threatening respiratory depression has been reported with the use of opioids, even when used as recommended, especially in patients with chronic pulmonary disease, or in elderly, cachectic and debilitated patients. The risk is greatest during initiation of OLINVYK therapy, following a dose increase, or when used with other drugs that depress respiration. Proper dosing of OLINVYK is essential, especially when converting patients from another opioid product to avoid overdose. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status. • Opioids can cause sleep - related breathing disorders including central sleep apnea (CSA) and sleep - related hypoxemia with risk increasing in a dose - dependent fashion. In patients who present with CSA, consider decreasing the dose of opioid using best practices for opioid taper. INDICATIONS AND USAGE OLINVYK is a new chemical entity indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. 21 |
| WARNINGS AND PRECAUTIONS • Prolonged use of opioids during pregnancy can result in withdrawal in the neonate that may be life - threatening. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using OLINVYK for a prolonged period of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. • P r o f ound s e d a tion, re spi r a to r y d e p r e ssion, c om a , a nd d ea th m a y re sult f r om the c on c omit a nt use of OLINVYK with b e n z odi a z e pin e s or oth e r C NS d e p re ss a nts ( e .. g .., non - b e n z odi a z e pine s e d a tiv e s/ h y pnoti c s, a n x i o l y ti c s, t ra nquili z er s, mus c le re l a x a nts, g e n e ra l a n e sth e ti c s, a ntip s y c hoti c s, oth e r opioids, or a l c ohol ) .. B e ca u s e o f th e se r isks, re s er ve c o n c omit a nt p re s cr ibi n g of th e se d r u g s f o r use in p a ti e nts f or whom a lt er n a tive t rea tm e nt options a r e in a d e qu a t e , prescribe the lowest effective dose, and minimize the duration. • OLINVYK was shown to have mild QTc interval prolongation in thorough QT studies where patients were dosed up to 27 mg. Total cumulative daily doses exceeding 27 mg per day were not studied and may increase the risk for QTc interval prolongation. Therefore, the cumulative total daily dose of OLINVYK should not exceed 27 mg. • Increased plasma concentrations of OLINVYK may occur in patients with decreased Cytochrome P450 (CYP) 2D6 function or normal metabolizers taking moderate or strong CYP2D6 inhibitors ; also in patients taking a moderate or strong CYP3A4 inhibitor, in patients with decreased CYP2D6 function who are also receiving a moderate or strong CYP3A4 inhibitor, or with discontinuation of a CYP3A4 inducer. These patients may require less frequent dosing and should be closely monitored for respiratory depression and sedation at frequent intervals. Concomitant use of OLINVYK with CYP3A4 inducers or discontinuation of a moderate or strong CYP3A4 inhibitor can lower the expected concentration, which may decrease efficacy, and may require supplemental doses. • Cases of adrenal insufficiency have been reported with opioid use (usually greater than one month). Presentation and symptoms may be nonspecific and include nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If confirmed, treat with physiologic replacement doses of corticosteroids and wean patient from the opioid. • OLINVYK m a y ca u s e s e v e r e h y po t e ns i o n, i n c l u d i n g or t h o s tatic h y po t e ns i o n a n d s yn c op e in a m b u lat o r y p atie n t s .. • T h e r e is i n c r ea s ed r i s k in p ati e n ts w ho s e a b ili t y to m a i n ta i n b l oo d pr e ssu r e h as a l r ea d y b een c o m pr o m i s ed b y a r e d u ced b l oo d v o l u m e o r c o n c u rr e n t a d m i n i s t r ati o n o f c e r tain C N S d e pr e ss a n t dru g s ( e .. g .. , phenothiazines or g e n e r al a n e s t h eti c s ). M o n it o r t h e s e p atie n ts f o r s i g n s o f h y po t e ns i o n. I n p atie n t s w i t h ci r c u lat o r y s h o c k , avoid the use of OLINVYK as it m a y c a us e v a s od ilati o n t h at can f u r t h er r e d u ce ca rd iac o u t p u t a n d b l oo d pr e ssu r e. • A v o id t h e us e o f OLINVYK in p atie n ts w i th i m p ai r ed c o ns ci o u s n e s s o r c o m a. OLINVYK should be used with caution in p atie n ts w h o m a y b e s us ce p ti b le to t h e i n t r ac r a n ial e f f ec t s o f C O 2 r et e n ti o n, such as t h o s e with e v i d e n ce o f i n c r ea s ed i n t r ac r a n ial pr e s su r e o r br ain t u m or s , as a reduction in r e s p i r at or y dr i v e a n d t h e r e su lt a n t C O 2 r et e n ti o n can f u r t h er i n c r ea s e i n t r ac r a n i al pr e ssu r e. Mo n it o r su c h p at i e n ts f o r s i g n s o f s e d ati o n a n d r e s p i r at or y d e pr e ss i o n , p a r tic u la r l y w h en i n i t iat i n g t h e r a p y. • As with all opioids, OLINVYK m a y ca u s e s p a s m o f t h e s p h i n c ter o f O dd i , and m a y ca us e i n c r ea s es i n s e r u m a my la s e. Mo n it o r p atie n ts w i t h b ilia r y t r act d i s ea s e, i n c l u d i n g a c u te p a n c r eatit i s , f o r w o r s e n i n g s y m p t o ms .. • T h e r e is i n c r ea s ed r i s k in p ati e n ts w ho s e a b ili t y to m a i n ta i n b l oo d pr e ssu r e h as a l r ea d y b een c o m pr o m i s ed b y a r e d u ced b l oo d v o l u m e o r c o n c u rr e n t a d m i n i s t r ati o n o f c e r tain C N S d e pr e ss a n t dru g s ( e .. g .. , phenothiazines or g e n e r al a n e s t h eti c s ). M o n it o r t h e s e p atie n ts f o r s i g n s o f h y po t e ns i o n. I n p atie n t s w i t h ci r c u lat o r y s h o c k , avoid the use of OLINVYK as it m a y c a us e v a s od ilati o n t h at can f u r t h er r e d u ce ca rd iac o u t p u t a n d b l oo d pr e ssu r e. • A v o id t h e us e o f OLINVYK in p atie n ts w i th i m p ai r ed c o ns ci o u s n e s s o r c o m a. OLINVYK should be used with caution in p atie n ts w h o m a y b e s us ce p ti b le to t h e i n t r ac r a n ial e f f ec t s o f C O 2 r et e n ti o n, such as t h o s e with e v i d e n ce o f i n c r ea s ed i n t r ac r a n ial pr e s su r e o r br ain t u m or s , as a reduction in r e s p i r at or y dr i v e a n d t h e r e su lt a n t C O 2 r et e n ti o n can f u r t h er i n c r ea s e i n t r ac r a n i al pr e ssu r e. Mo n it o r su c h p at i e n ts f o r s i g n s o f s e d ati o n a n d r e s p i r at or y d e pr e ss i o n , p a r tic u la r l y w h en i n i t iat i n g t h e r a p y. • As with all opioids, OLINVYK m a y ca u s e s p a s m o f t h e s p h i n c ter o f O dd i , and m a y ca us e i n c r ea s es i n s e r u m a my la s e. Mo n it o r p atie n ts w i t h b ilia r y t r act d i s ea s e, i n c l u d i n g a c u te p a n c r eatit i s , f o r w o r s e n i n g s y m p t o ms .. • OLINVYK m a y i n c r ea s e t h e f r e q u e n c y o f s ei z u r es i n p atie n t s w i t h s eiz u r e d i s ord e r s a n d may increase t h e r i s k o f s ei z u r es in vulnerable patients .. M o n it o r p atie n ts w i th a h i s t o r y o f s ei z u r e d i s ord e r s f o r w or s e n ed s eiz u r e c o n t ro l. • Do not abruptly discontinue OLINVYK in a patient physically dependent on opioids. Gradually taper the dosage to avoid a withdrawal syndrome and return of pain. Avoid the use of mixed agonist/antagonist (e.g., pentazocine , nalbuphine , and butorphanol ) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving OLINVYK, as they may reduce the analgesic effect and/or precipitate withdrawal symptoms. • OLINVYK may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. • Although self - administration of opioids by patient - controlled analgesia (PCA) may allow each patient to individually titrate to an acceptable level of analgesia, PCA administration has resulted in adverse outcomes and episodes of respiratory depression. Health care providers and family members monitoring patients receiving PCA analgesia should be instructed in the need for appropriate monitoring for excessive sedation, respiratory depression, or other adverse effects of opioid medications. ADVERSE REACTIONS Adverse reactions are described in greater detail in the Prescribing Information. The most common (incidence ≥10%) adverse reactions in Phase 3 controlled clinical trials were nausea, vomiting, dizziness, headache, constipation, pruritus, and hypoxia. PLEASE see www.OLNVYK.com for full prescribing information including BOXED warning and important safety information 22 |