EXHIBIT 99.1
Published on October 2, 2023
|
INNOVATING FOR PATIENTS Nasdaq: TRVN I October 2023 |
|
Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts regarding Trevena, Inc. (the “Company” or “we”), they are forward-looking statements reflecting management’s current beliefs and expectations. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “objective,” “predict,” “project,” “suggest,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “ongoing,” or the negative of these terms or similar expressions. Forward-looking statements contained in this presentation include, but are not limited to, (i) statements regarding the timing of anticipated clinical trials for our product candidates; (ii) the timing of receipt of clinical data for our product candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the size of patient populations targeted by our product candidates and market adoption of our potential drugs by physicians and patients; (v) the timing or likelihood of regulatory filings and approvals; and (vi) our cash needs. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the commercialization of any approved drug product, the status, timing, costs, results and interpretation of our clinical trials or any future trials of any of our investigational drug candidates; the uncertainties inherent in conducting clinical trials; expectations for regulatory interactions, submissions and approvals, including our assessment of the discussions with the FDA or other regulatory agencies about any and all of our programs; uncertainties related to the commercialization of OLINVYK; available funding; uncertainties related to our intellectual property; other matters that could affect the availability or commercial potential of our therapeutic candidates; and other factors discussed in the Risk Factors set forth in our Annual Report on Form 10-K and Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission (SEC) and in other filings we make with the SEC from time to time. In addition, the forward-looking statements included in this presentation represent our views only as of the date hereof. We anticipate that subsequent events and developments may cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so, except as may be required by law. 2 |
|
BOARD OF DIRECTORS Leon O. Moulder, Jr. Chairman Marvin H. Johnson, Jr. Carrie L. Bourdow Jake R. Nunn Scott Braunstein, M.D. Anne M. Phillips, M.D. Mark Corrigan, M.D. Barbara Yanni SENIOR MANAGEMENT Carrie L. Bourdow President & Chief Executive Officer Mark A. Demitrack, M.D. SVP, Chief Medical Officer Patricia Drake SVP, Chief Commercial Officer Barry Shin SVP, Chief Financial Officer Robert T. Yoder SVP, Chief Business Officer & Head of Commercial Operations Trevena’s Experienced Leadership Team 3 |
|
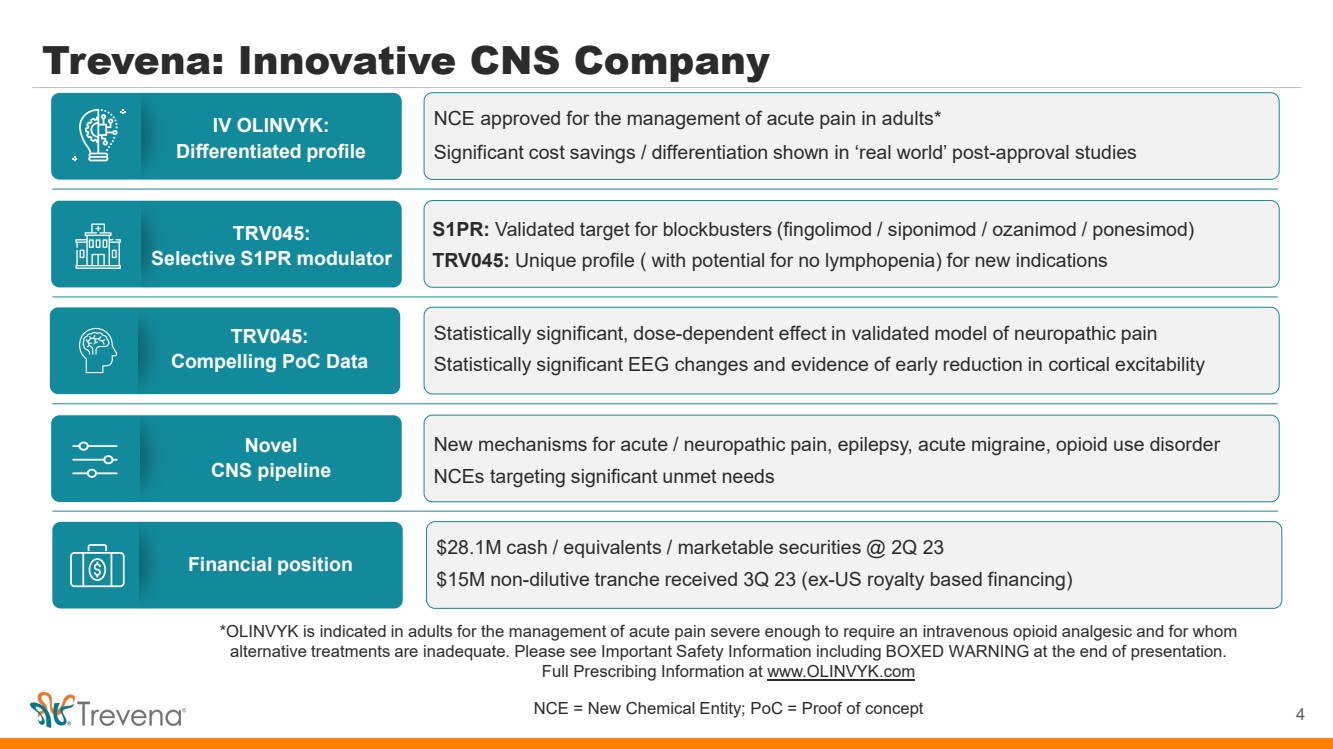
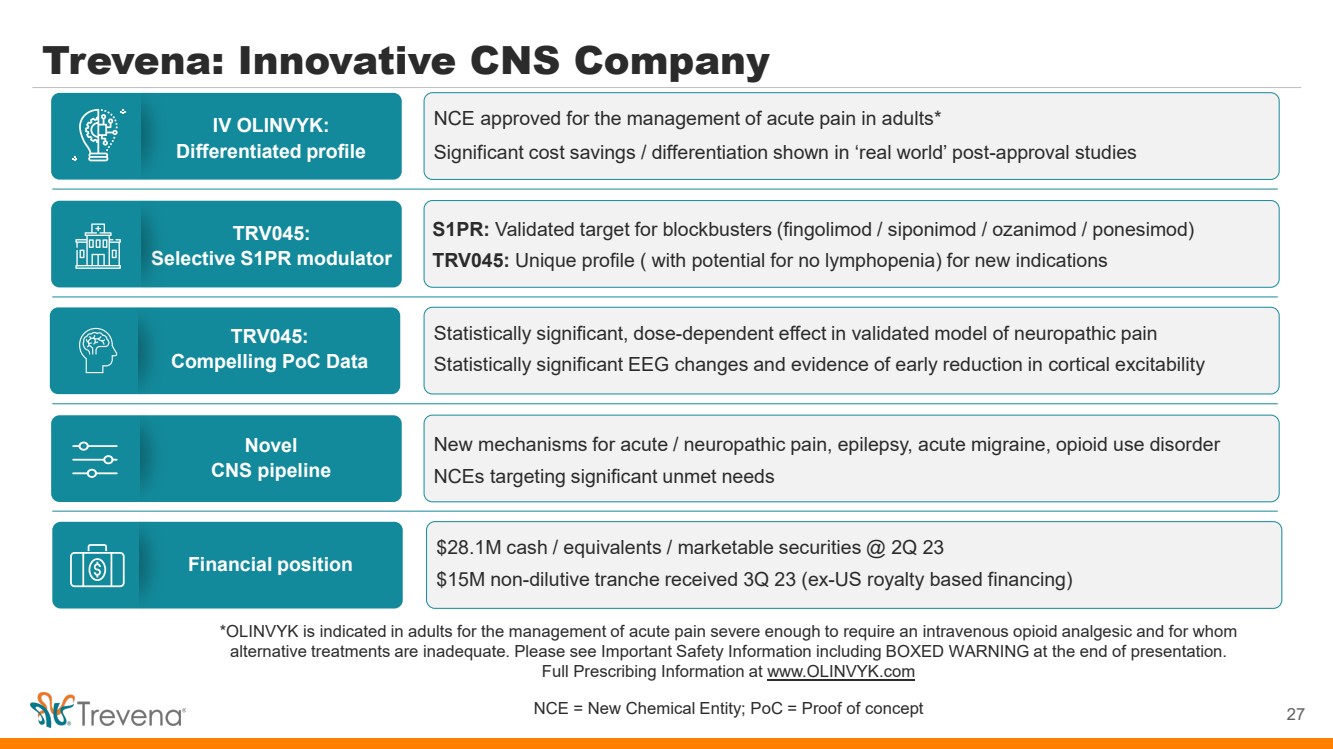
*OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com NCE = New Chemical Entity; PoC = Proof of concept Trevena: Innovative CNS Company 4 IV OLINVYK: Differentiated profile TRV045: Selective S1PR modulator Novel CNS pipeline TRV045: Compelling PoC Data Financial position NCE approved for the management of acute pain in adults* Significant cost savings / differentiation shown in ‘real world’ post-approval studies S1PR: Validated target for blockbusters (fingolimod / siponimod / ozanimod / ponesimod) TRV045: Unique profile ( with potential for no lymphopenia) for new indications New mechanisms for acute / neuropathic pain, epilepsy, acute migraine, opioid use disorder NCEs targeting significant unmet needs Statistically significant, dose-dependent effect in validated model of neuropathic pain Statistically significant EEG changes and evidence of early reduction in cortical excitability $28.1M cash / equivalents / marketable securities @ 2Q 23 $15M non-dilutive tranche received 3Q 23 (ex-US royalty based financing) |
|
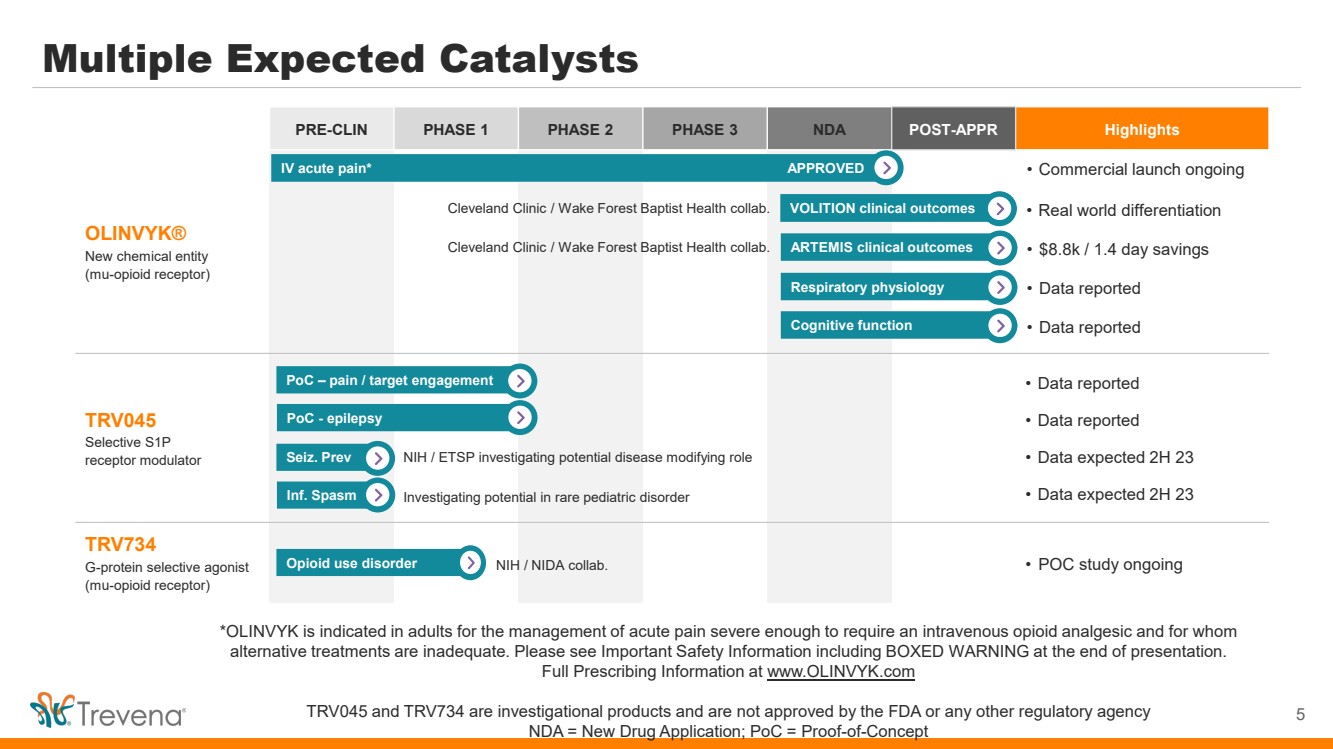
*OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com TRV045 and TRV734 are investigational products and are not approved by the FDA or any other regulatory agency NDA = New Drug Application; PoC = Proof-of-Concept PRE-CLIN PHASE 1 PHASE 2 PHASE 3 NDA POST-APPR Highlights OLINVYK® New chemical entity (mu-opioid receptor) TRV045 Selective S1P receptor modulator • Data reported • Data reported • Data expected 2H 23 • Data expected 2H 23 TRV734 G-protein selective agonist (mu-opioid receptor) Multiple Expected Catalysts 5 NIH / NIDA collab. IV acute pain* VOLITION clinical outcomes Respiratory physiology Cleveland Clinic / Wake Forest Baptist Health collab. PoC – pain / target engagement PoC - epilepsy APPROVED • Real world differentiation • Data reported • Commercial launch ongoing • POC study ongoing ARTEMIS clinical outcomes • $8.8k / 1.4 day savings Opioid use disorder Seiz. Prev Inf. Spasm NIH / ETSP investigating potential disease modifying role Investigating potential in rare pediatric disorder Cognitive function Cleveland Clinic / Wake Forest Baptist Health collab. • Data reported |
|
OLINVYK Overview |
|
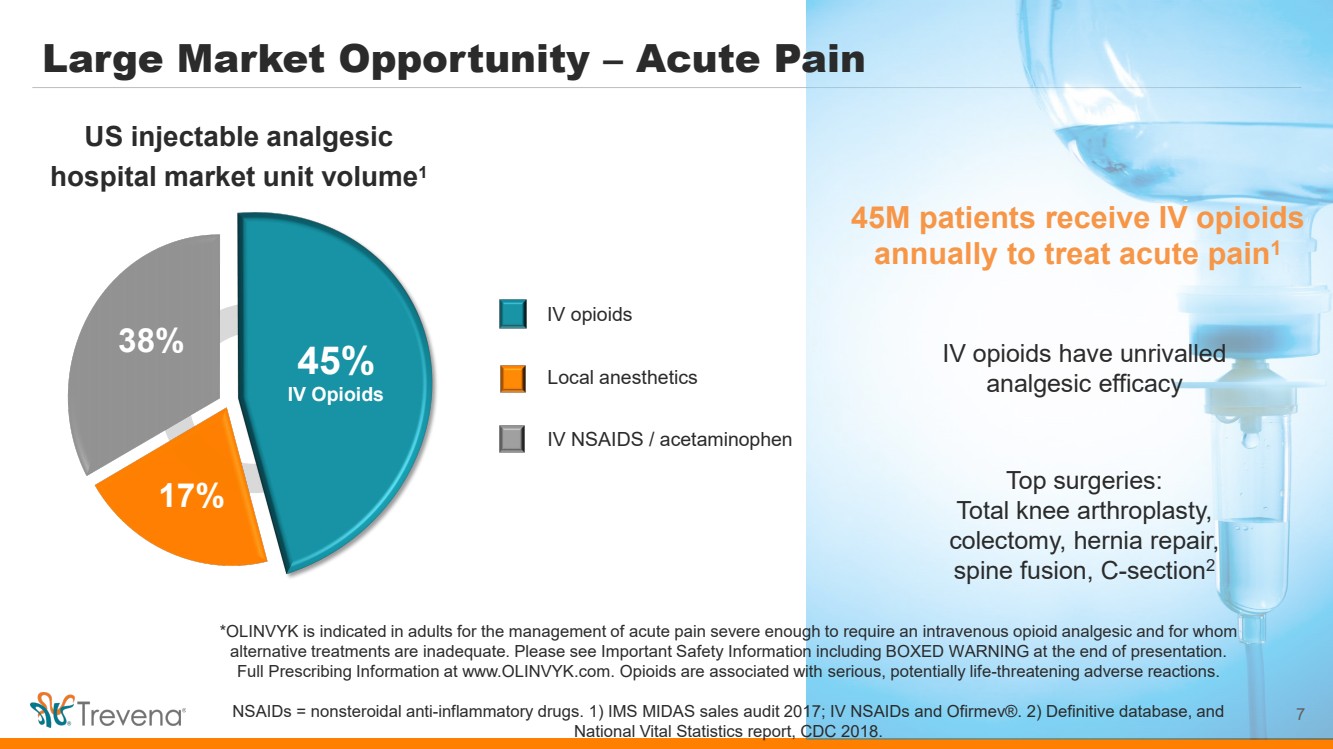
Large Market Opportunity – Acute Pain 7 45M patients receive IV opioids annually to treat acute pain1 IV opioids have unrivalled analgesic efficacy Top surgeries: Total knee arthroplasty, colectomy, hernia repair, spine fusion, C-section2 IV NSAIDS / acetaminophen US injectable analgesic hospital market unit volume1 IV opioids 45% IV Opioids 17% 38% Local anesthetics *OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com. Opioids are associated with serious, potentially life-threatening adverse reactions. NSAIDs = nonsteroidal anti-inflammatory drugs. 1) IMS MIDAS sales audit 2017; IV NSAIDs and Ofirmev®. 2) Definitive database, and National Vital Statistics report, CDC 2018. |
|
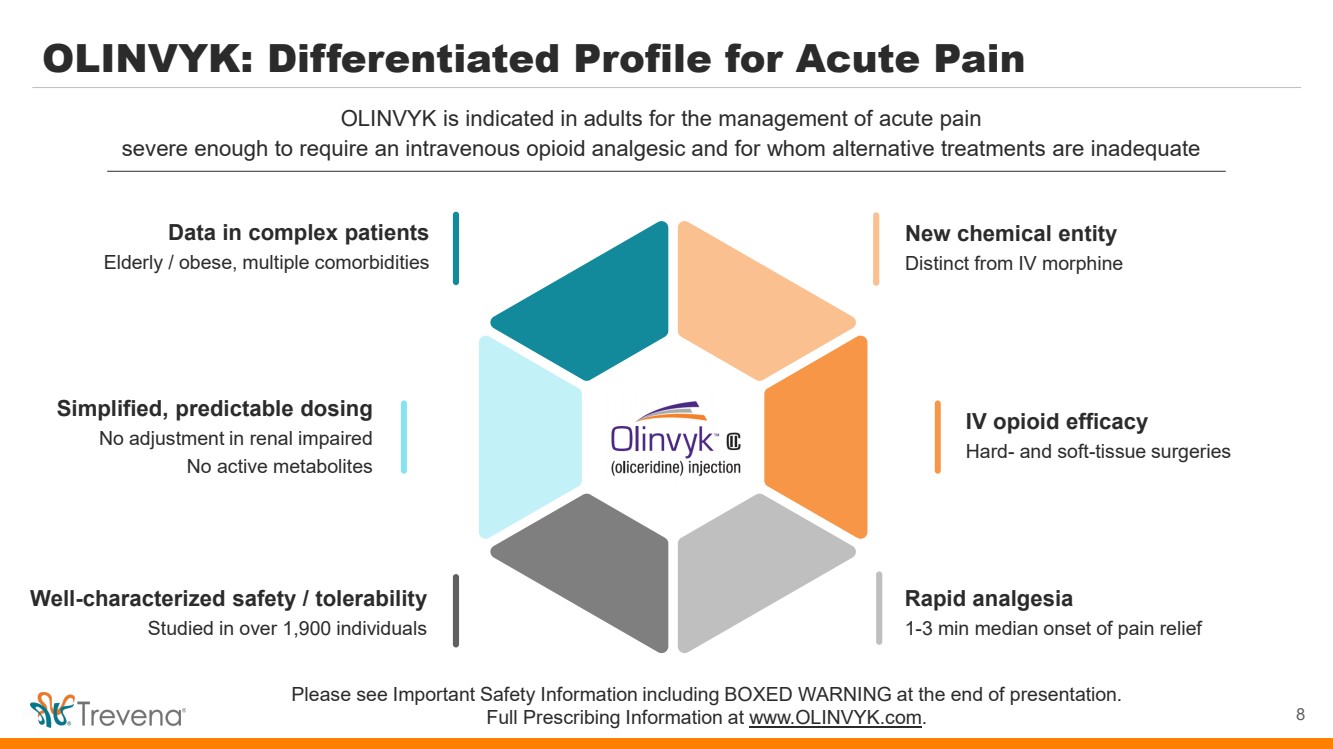
OLINVYK: Differentiated Profile for Acute Pain 8 OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com. New chemical entity Distinct from IV morphine IV opioid efficacy Hard- and soft-tissue surgeries Rapid analgesia 1-3 min median onset of pain relief Simplified, predictable dosing No adjustment in renal impaired No active metabolites Data in complex patients Elderly / obese, multiple comorbidities Well-characterized safety / tolerability Studied in over 1,900 individuals |
|
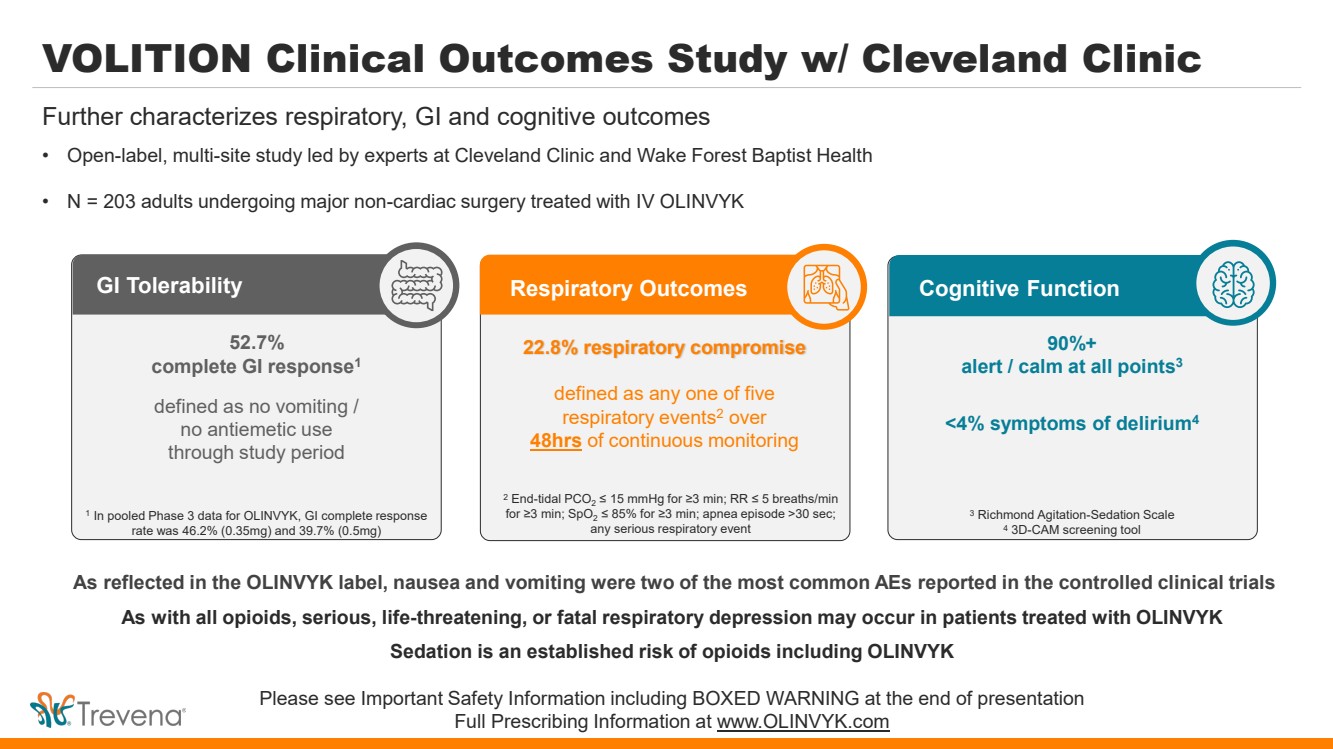
VOLITION Clinical Outcomes Study w/ Cleveland Clinic • Open-label, multi-site study led by experts at Cleveland Clinic and Wake Forest Baptist Health • N = 203 adults undergoing major non-cardiac surgery treated with IV OLINVYK Further characterizes respiratory, GI and cognitive outcomes Cognitive Function 90%+ alert / calm at all points3 <4% symptoms of delirium4 3 Richmond Agitation-Sedation Scale 4 3D-CAM screening tool As reflected in the OLINVYK label, nausea and vomiting were two of the most common AEs reported in the controlled clinical trials As with all opioids, serious, life-threatening, or fatal respiratory depression may occur in patients treated with OLINVYK Sedation is an established risk of opioids including OLINVYK Please see Important Safety Information including BOXED WARNING at the end of presentation Full Prescribing Information at www.OLINVYK.com GI Tolerability 52.7% complete GI response1 defined as no vomiting / no antiemetic use through study period 1 In pooled Phase 3 data for OLINVYK, GI complete response rate was 46.2% (0.35mg) and 39.7% (0.5mg) Respiratory Outcomes 22.8% respiratory compromise defined as any one of five respiratory events2 over 48hrs of continuous monitoring 2 End-tidal PCO2 ≤ 15 mmHg for ≥3 min; RR ≤ 5 breaths/min for ≥3 min; SpO2 ≤ 85% for ≥3 min; apnea episode >30 sec; any serious respiratory event |
|
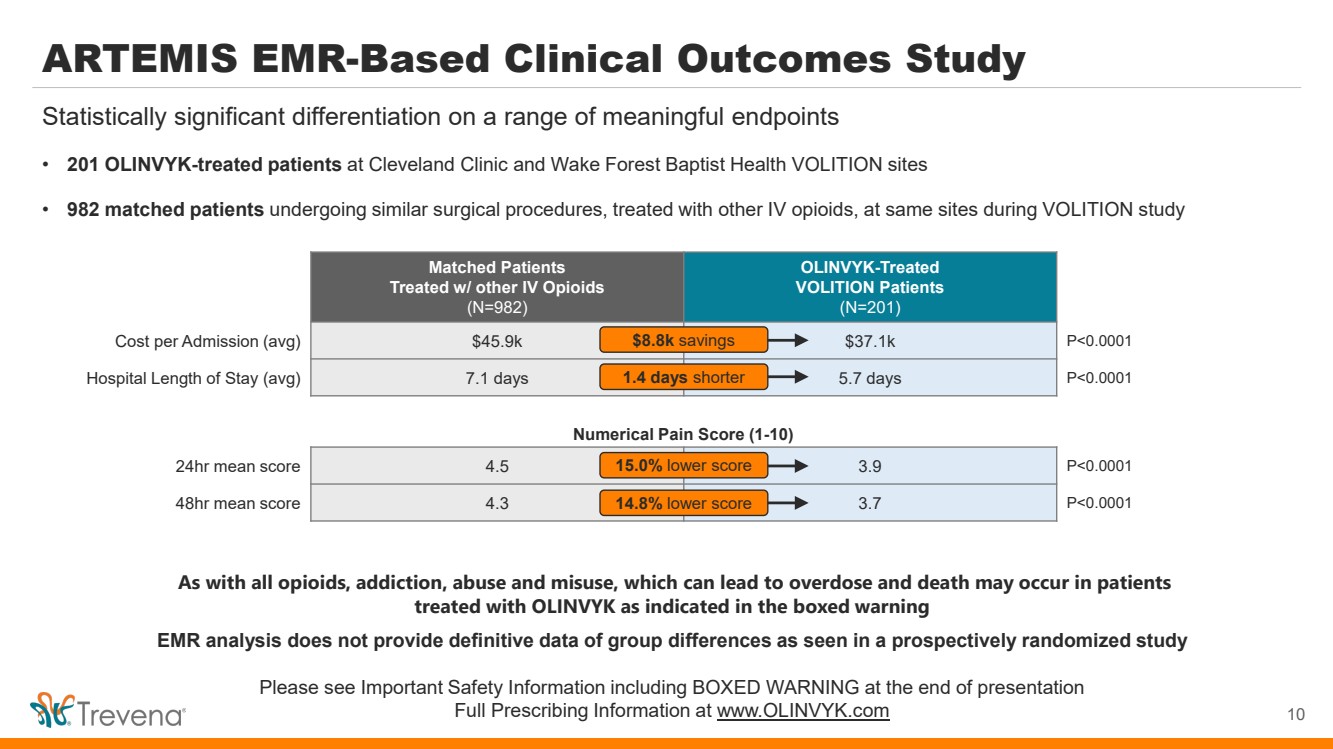
ARTEMIS EMR-Based Clinical Outcomes Study • 201 OLINVYK-treated patients at Cleveland Clinic and Wake Forest Baptist Health VOLITION sites • 982 matched patients undergoing similar surgical procedures, treated with other IV opioids, at same sites during VOLITION study 10 Statistically significant differentiation on a range of meaningful endpoints Matched Patients Treated w/ other IV Opioids (N=982) OLINVYK-Treated VOLITION Patients (N=201) Cost per Admission (avg) $45.9k $37.1k P<0.0001 Hospital Length of Stay (avg) 7.1 days 5.7 days P<0.0001 $8.8k savings 1.4 days shorter 24hr mean score 4.5 3.9 P<0.0001 48hr mean score 4.3 3.7 P<0.0001 15.0% lower score 14.8% lower score Numerical Pain Score (1-10) As with all opioids, addiction, abuse and misuse, which can lead to overdose and death may occur in patients treated with OLINVYK as indicated in the boxed warning EMR analysis does not provide definitive data of group differences as seen in a prospectively randomized study Please see Important Safety Information including BOXED WARNING at the end of presentation Full Prescribing Information at www.OLINVYK.com |
|
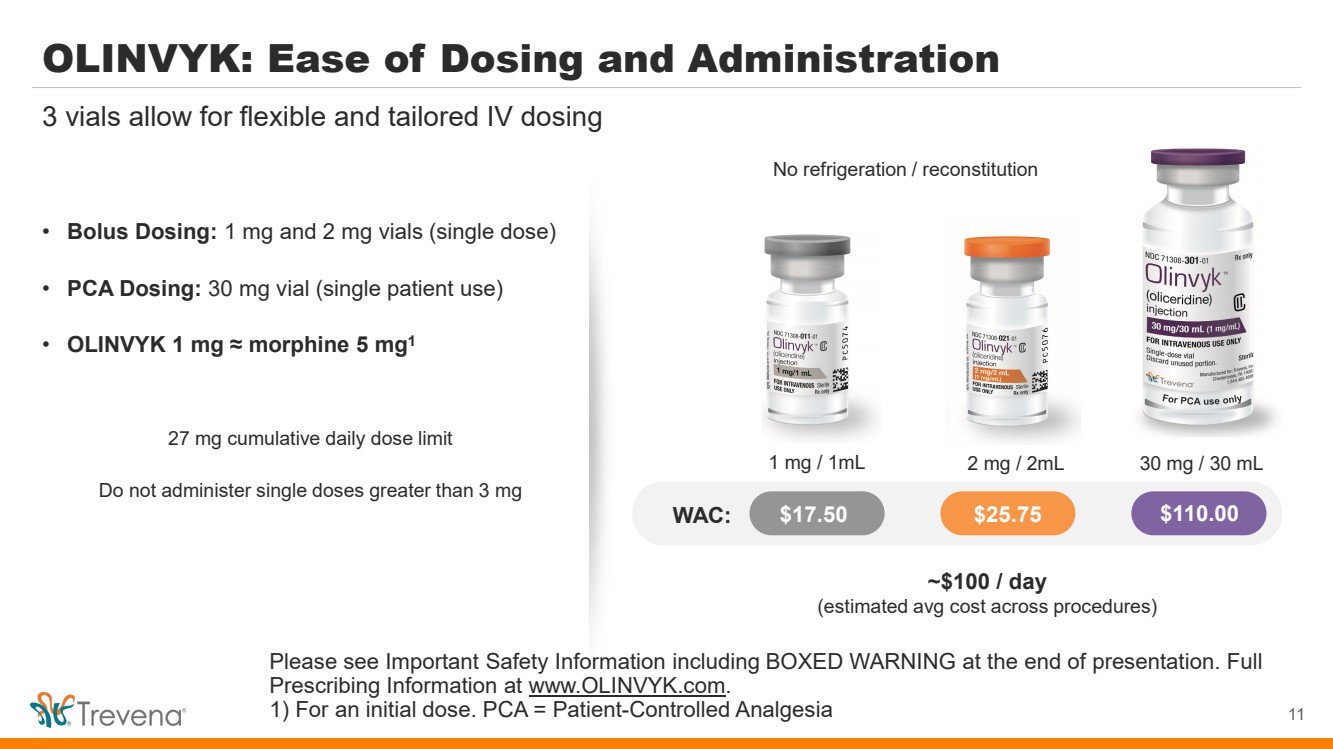
OLINVYK: Ease of Dosing and Administration • Bolus Dosing: 1 mg and 2 mg vials (single dose) • PCA Dosing: 30 mg vial (single patient use) • OLINVYK 1 mg ≈ morphine 5 mg1 27 mg cumulative daily dose limit Do not administer single doses greater than 3 mg 11 3 vials allow for flexible and tailored IV dosing No refrigeration / reconstitution WAC: $17.50 $25.75 $110.00 1 mg / 1mL 2 mg / 2mL 30 mg / 30 mL ~$100 / day (estimated avg cost across procedures) Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com. 1) For an initial dose. PCA = Patient-Controlled Analgesia |
|
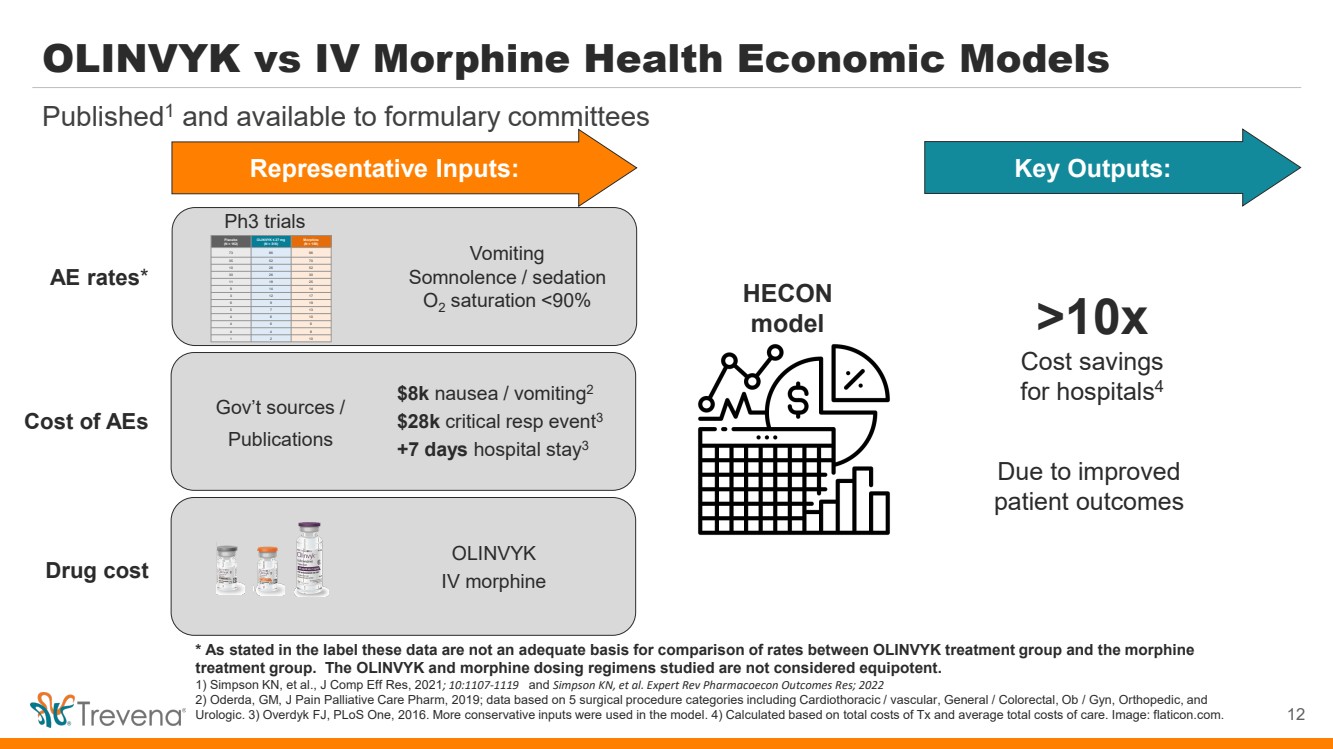
OLINVYK vs IV Morphine Health Economic Models 12 Published1 and available to formulary committees * As stated in the label these data are not an adequate basis for comparison of rates between OLINVYK treatment group and the morphine treatment group. The OLINVYK and morphine dosing regimens studied are not considered equipotent. 1) Simpson KN, et al., J Comp Eff Res, 2021; 10:1107-1119 and Simpson KN, et al. Expert Rev Pharmacoecon Outcomes Res; 2022 2) Oderda, GM, J Pain Palliative Care Pharm, 2019; data based on 5 surgical procedure categories including Cardiothoracic / vascular, General / Colorectal, Ob / Gyn, Orthopedic, and Urologic. 3) Overdyk FJ, PLoS One, 2016. More conservative inputs were used in the model. 4) Calculated based on total costs of Tx and average total costs of care. Image: flaticon.com. Vomiting Somnolence / sedation O2 saturation <90% Representative Inputs: >10x Cost savings for hospitals4 Due to improved patient outcomes HECON model Placebo (N = 162) OLINVYK ≤ 27 mg (N = 316) Morphine (N = 158) 73 86 96 35 52 70 10 26 52 30 26 30 11 18 25 9 14 14 3 12 17 6 9 19 5 7 13 4 6 10 4 6 6 4 4 8 1 2 10 AE rates* Cost of AEs Drug cost Ph3 trials Gov’t sources / Publications $8k nausea / vomiting2 $28k critical resp event3 +7 days hospital stay3 OLINVYK IV morphine Key Outputs: |
|
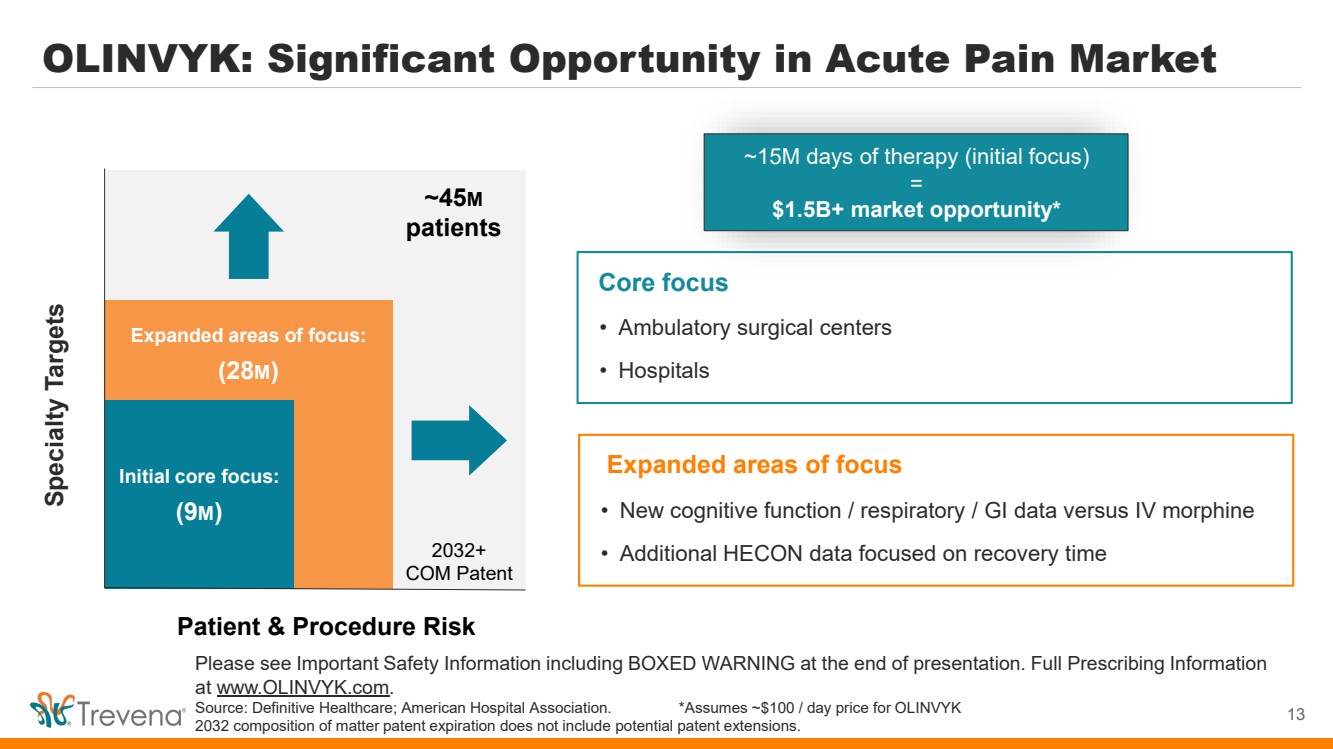
OLINVYK: Significant Opportunity in Acute Pain Market 13 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com. Source: Definitive Healthcare; American Hospital Association. *Assumes ~$100 / day price for OLINVYK 2032 composition of matter patent expiration does not include potential patent extensions. Specialty Targets Patient & Procedure Risk Initial launch focus ~45M patients Initial core focus: (9M) Expanded areas of focus: (28M) • Ambulatory surgical centers • Hospitals Core focus ~15M days of therapy (initial focus) = $1.5B+ market opportunity* • New cognitive function / respiratory / GI data versus IV morphine • Additional HECON data focused on recovery time Expanded areas of focus 2032+ COM Patent |
|
TRV045 S1P Receptor Modulator Novel MOA for Diabetic Neuropathic Pain |
|
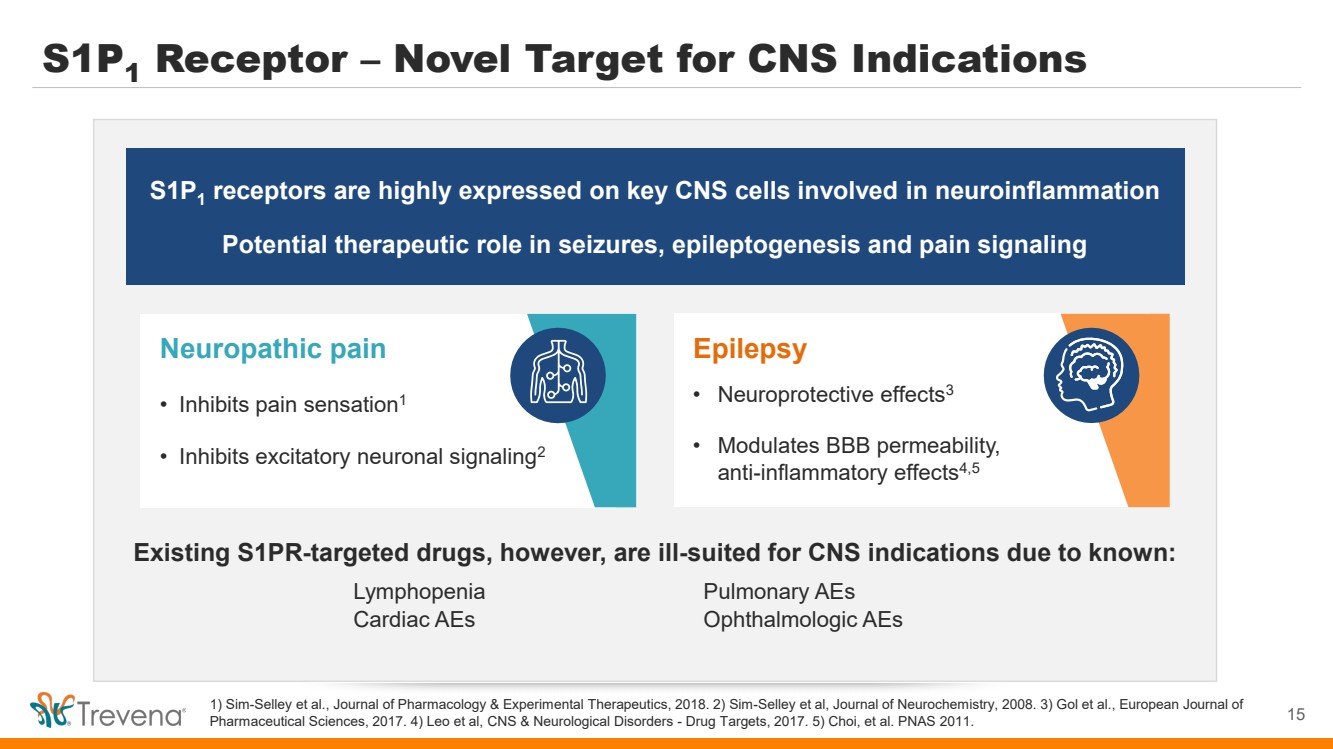
Epilepsy • Neuroprotective effects3 • Modulates BBB permeability, anti-inflammatory effects4,5 Neuropathic pain • Inhibits pain sensation1 • Inhibits excitatory neuronal signaling2 S1P1 Receptor – Novel Target for CNS Indications 1) Sim-Selley et al., Journal of Pharmacology & Experimental Therapeutics, 2018. 2) Sim-Selley et al, Journal of Neurochemistry, 2008. 3) Gol et al., European Journal of Pharmaceutical Sciences, 2017. 4) Leo et al, CNS & Neurological Disorders - Drug Targets, 2017. 5) Choi, et al. PNAS 2011. S1P1 receptors are highly expressed on key CNS cells involved in neuroinflammation Potential therapeutic role in seizures, epileptogenesis and pain signaling Existing S1PR-targeted drugs, however, are ill-suited for CNS indications due to known: Lymphopenia Pulmonary AEs Cardiac AEs Ophthalmologic AEs 15 |
|
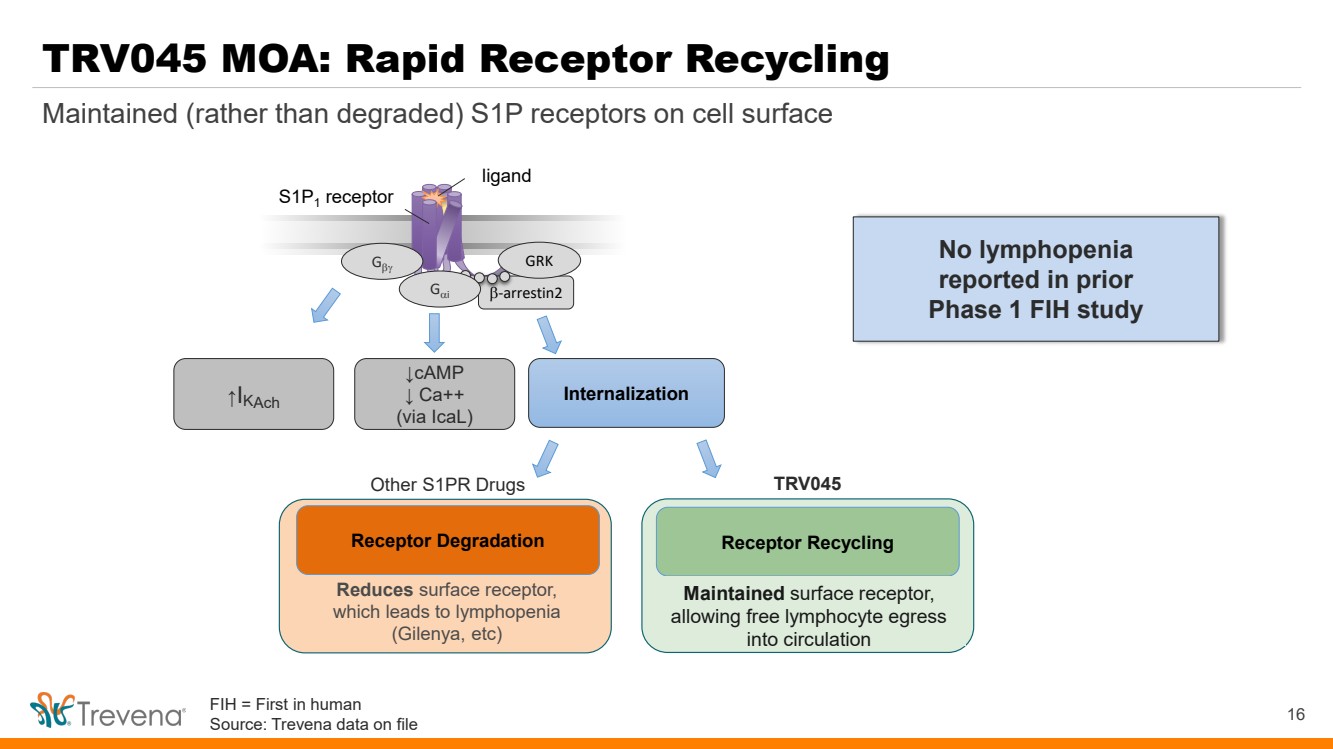
TRV045 MOA: Rapid Receptor Recycling β-arrestin2 S1P1 receptor Gβγ GRK ↑I Internalization KAch ligand Gαi ↓cAMP ↓ Ca++ (via IcaL) Reduces surface receptor, which leads to lymphopenia (Gilenya, etc) Receptor Recycling Maintains surface receptor and avoids lymphopenia, allowing free lymphocyte egress into circulation Receptor Degradation Other S1PR Drugs TRV045 16 Maintained (rather than degraded) S1P receptors on cell surface No lymphopenia reported in prior Phase 1 FIH study Maintained surface receptor, allowing free lymphocyte egress into circulation FIH = First in human Source: Trevena data on file |
|
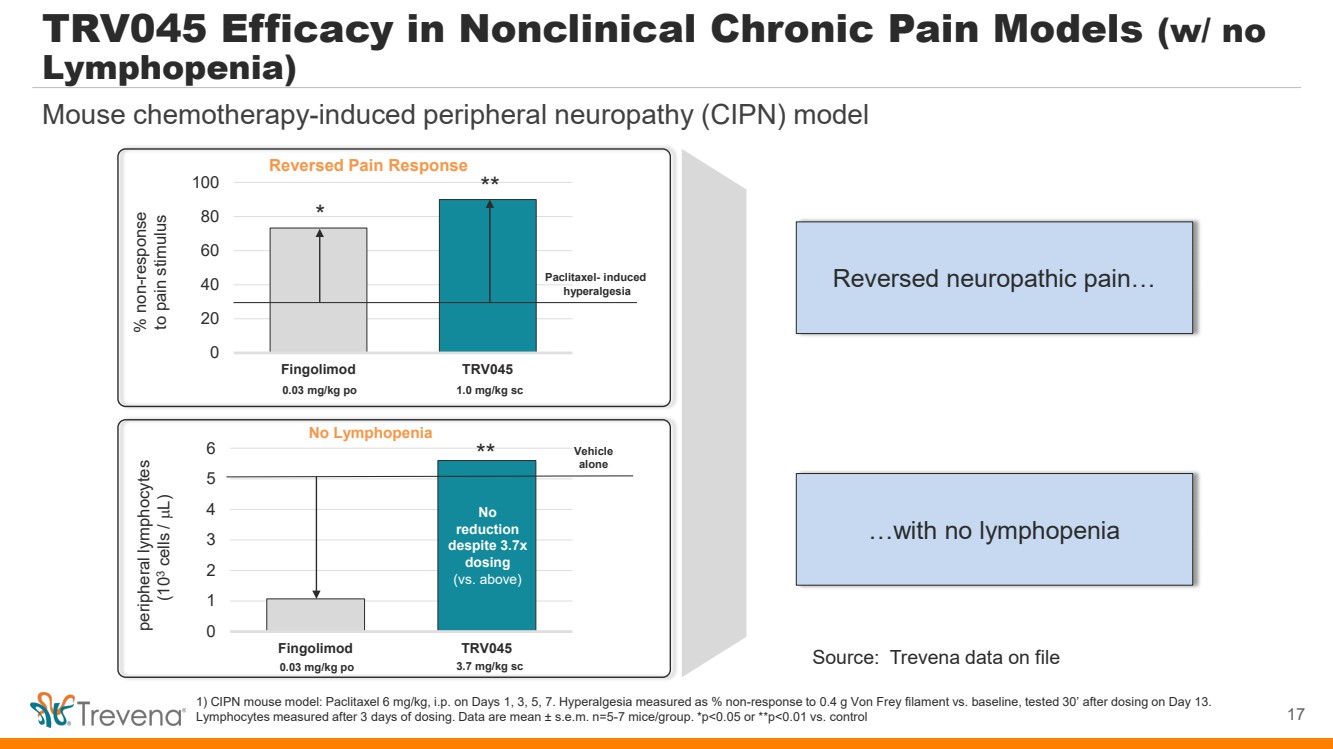
0 1 2 3 4 5 6 Fingolimod TRV045 No Lymphopenia TRV045 Efficacy in Nonclinical Chronic Pain Models (w/ no Lymphopenia) 1) CIPN mouse model: Paclitaxel 6 mg/kg, i.p. on Days 1, 3, 5, 7. Hyperalgesia measured as % non-response to 0.4 g Von Frey filament vs. baseline, tested 30’ after dosing on Day 13. Lymphocytes measured after 3 days of dosing. Data are mean ± s.e.m. n=5-7 mice/group. *p<0.05 or **p<0.01 vs. control peripheral lymphocytes (103 cells / µL) 0.03 mg/kg po 3.7 mg/kg sc Vehicle alone No reduction despite 3.7x dosing (vs. above) ** Reversed Pain Response 0 20 40 60 80 100 Fingolimod TRV045 % non-response to pain stimulus 0.03 mg/kg po 1.0 mg/kg sc Paclitaxel- induced hyperalgesia * ** 17 Mouse chemotherapy-induced peripheral neuropathy (CIPN) model Reversed neuropathic pain… …with no lymphopenia Source: Trevena data on file |
|
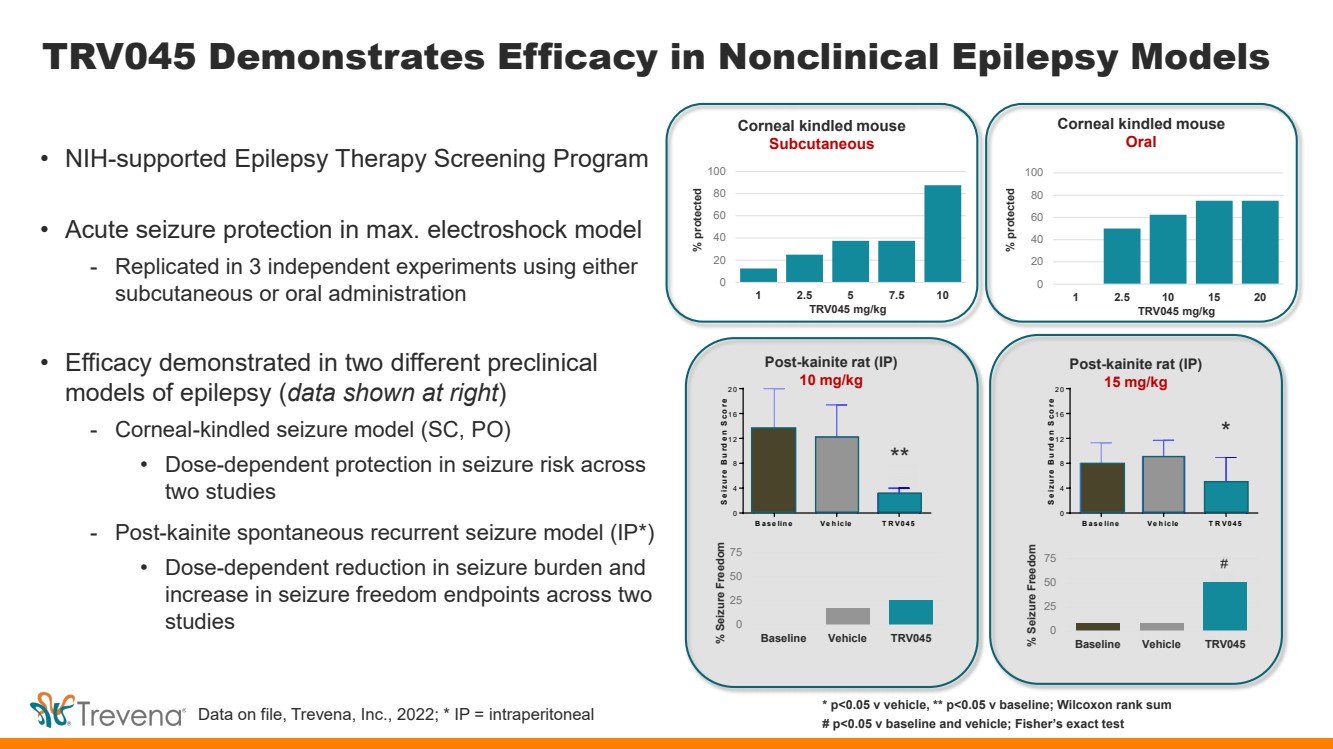
TRV045 Demonstrates Efficacy in Nonclinical Epilepsy Models • NIH-supported Epilepsy Therapy Screening Program • Acute seizure protection in max. electroshock model - Replicated in 3 independent experiments using either subcutaneous or oral administration • Efficacy demonstrated in two different preclinical models of epilepsy (data shown at right) - Corneal-kindled seizure model (SC, PO) • Dose-dependent protection in seizure risk across two studies - Post-kainite spontaneous recurrent seizure model (IP*) • Dose-dependent reduction in seizure burden and increase in seizure freedom endpoints across two studies B ase line Ve hicle T RV045 0 4 8 1 2 1 6 2 0 Seizure B urden S core * B ase line Ve hicle T RV045 0 4 8 1 2 1 6 2 0 Seizure B urden S core * * p<0.05 v vehicle, ** p<0.05 v baseline; Wilcoxon rank sum # p<0.05 v baseline and vehicle; Fisher’s exact test Corneal kindled mouse Subcutaneous Corneal kindled mouse Oral Post-kainite rat (IP) 10 mg/kg Post-kainite rat (IP) 15 mg/kg 0 20 40 60 80 100 1 2.5 5 7.5 10 0 20 40 60 80 100 1 2.5 10 15 20 % protected % protected TRV045 mg/kg TRV045 mg/kg Data on file, Trevena, Inc., 2022; * IP = intraperitoneal ** * # 0 25 50 75 % Seizure Freedom Baseline Vehicle TRV045 0 25 50 75 % Seizure Freedom Baseline Vehicle TRV045 |
|
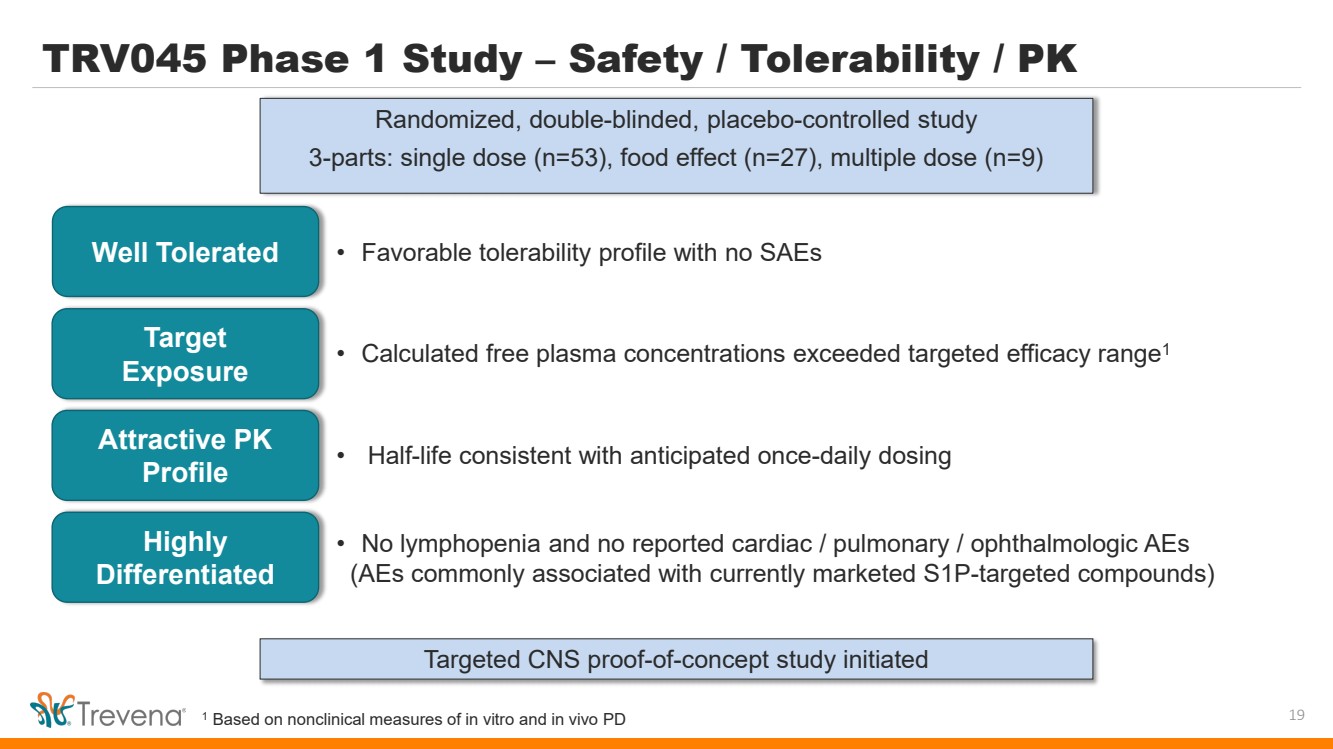
TRV045 Phase 1 Study – Safety / Tolerability / PK Randomized, double-blinded, placebo-controlled study 3-parts: single dose (n=53), food effect (n=27), multiple dose (n=9) 19 Well Tolerated • Favorable tolerability profile with no SAEs Target Exposure Attractive PK Profile • Calculated free plasma concentrations exceeded targeted efficacy range1 • Half-life consistent with anticipated once-daily dosing Targeted CNS proof-of-concept study initiated Highly Differentiated • No lymphopenia and no reported cardiac / pulmonary / ophthalmologic AEs (AEs commonly associated with currently marketed S1P-targeted compounds) 1 Based on nonclinical measures of in vitro and in vivo PD |
|
Studies were conducted outside the United States and not under the IND for TRV045 POC Studies: Target Engagement / TMS 20 Results confirm activity of central action and support advancement for neuropathic pain and other CNS indications Target Engagement Study Randomized, double-blind, placebo-controlled, 4x cross-over (n=25 subjects) Placebo or TRV045 (50/150/300mg) PainCart endpoints TMS Study Randomized, double-blind, placebo-controlled, multiple dose, 2x cross-over (n=25 subjects) Placebo or TRV045 (250mg / four days) EMG / EEG endpoints |
|
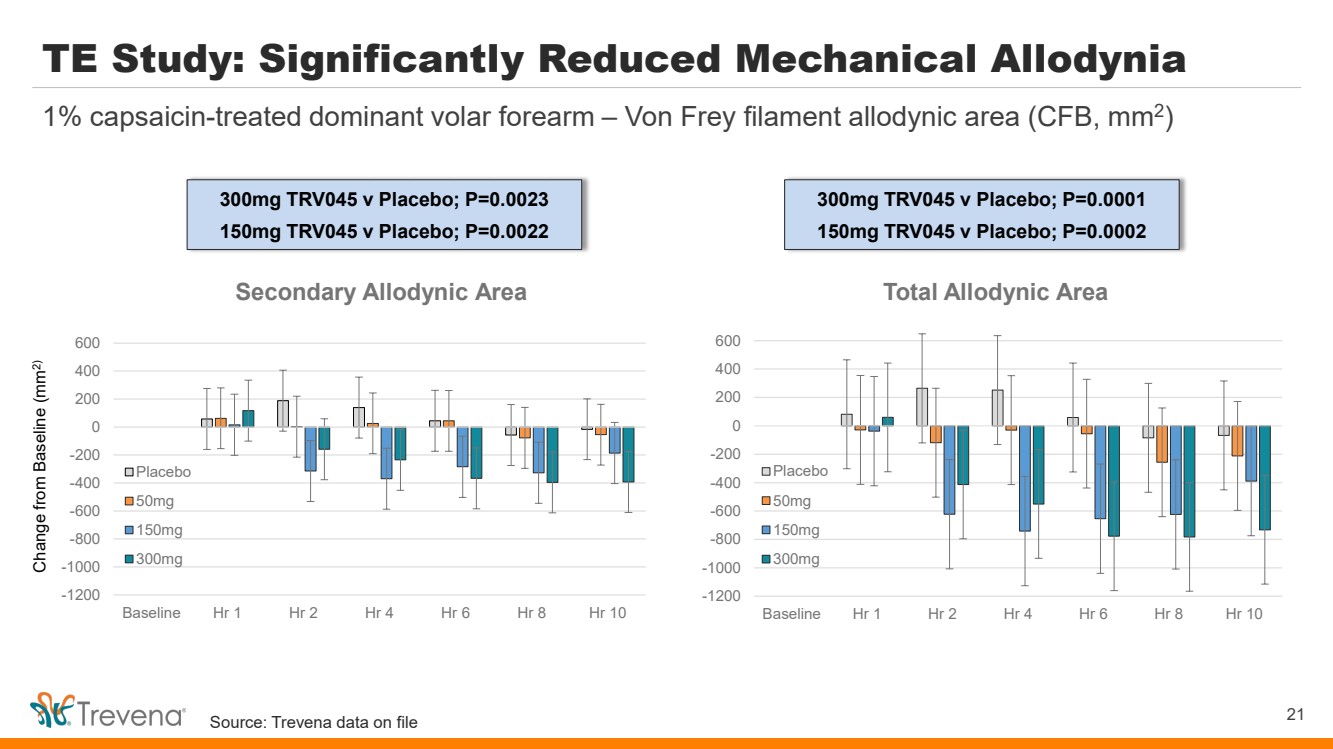
TE Study: Significantly Reduced Mechanical Allodynia 21 1% capsaicin-treated dominant volar forearm – Von Frey filament allodynic area (CFB, mm2) Change from Baseline (mm2) -1200 -1000 -800 -600 -400 -200 0 200 400 600 Baseline Hr 1 Hr 2 Hr 4 Hr 6 Hr 8 Hr 10 Total Allodynic Area Placebo 50mg 150mg 300mg -1200 -1000 -800 -600 -400 -200 0 200 400 600 Baseline Hr 1 Hr 2 Hr 4 Hr 6 Hr 8 Hr 10 Secondary Allodynic Area Placebo 50mg 150mg 300mg 300mg TRV045 v Placebo; P=0.0023 150mg TRV045 v Placebo; P=0.0022 300mg TRV045 v Placebo; P=0.0001 150mg TRV045 v Placebo; P=0.0002 Source: Trevena data on file |
|
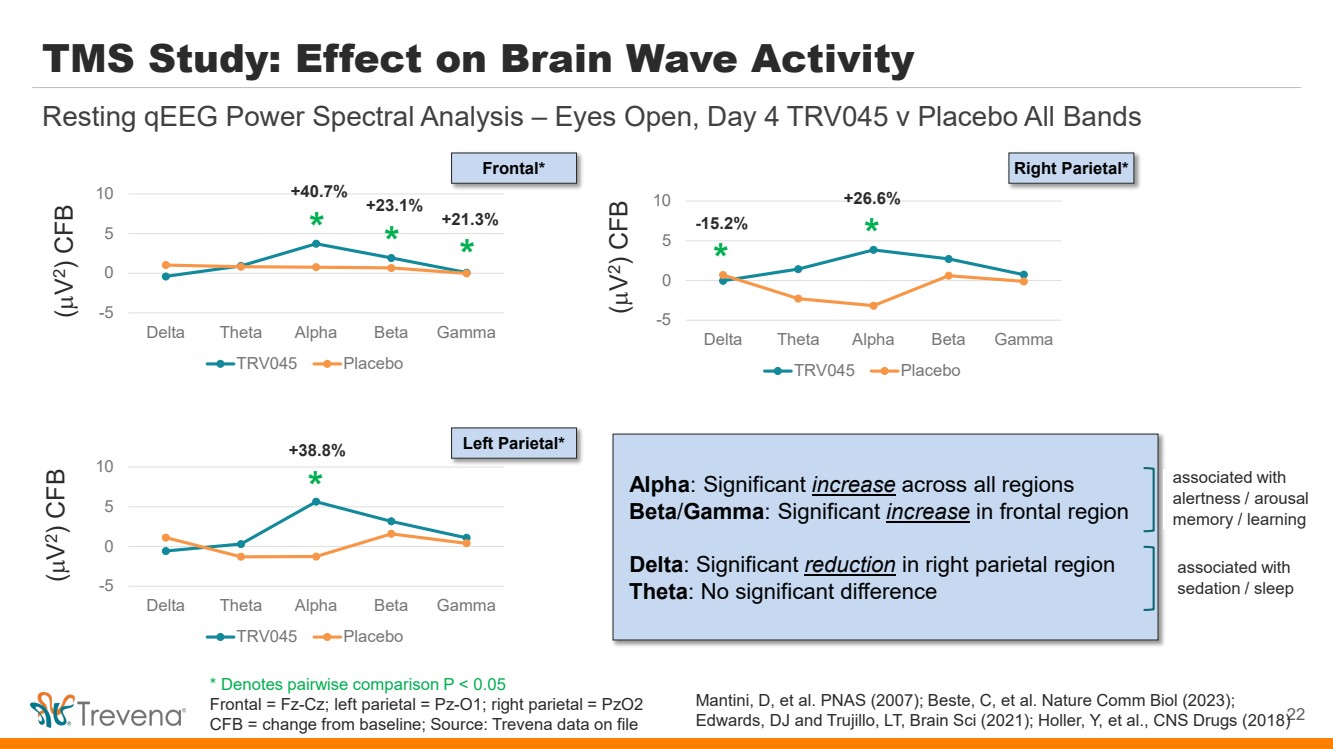
TMS Study: Effect on Brain Wave Activity -5 0 5 10 Delta Theta Alpha Beta Gamma TRV045 Placebo 22 Resting qEEG Power Spectral Analysis – Eyes Open, Day 4 TRV045 v Placebo All Bands -5 0 5 10 Delta Theta Alpha Beta Gamma TRV045 Placebo -5 0 5 10 Delta Theta Alpha Beta Gamma TRV045 Placebo * * * * * * +21.3% -15.2% +23.1% +40.7% +38.8% +26.6% (µ V 2) CFB (µ V 2) CFB (µ V 2) CFB Frontal* Left Parietal* Right Parietal* Alpha: Significant increase across all regions Beta/Gamma: Significant increase in frontal region Delta: Significant reduction in right parietal region Theta: No significant difference associated with alertness / arousal memory / learning associated with sedation / sleep Mantini, D, et al. PNAS (2007); Beste, C, et al. Nature Comm Biol (2023); Edwards, DJ and Trujillo, LT, Brain Sci (2021); Holler, Y, et al., CNS Drugs (2018) * Denotes pairwise comparison P < 0.05 Frontal = Fz-Cz; left parietal = Pz-O1; right parietal = PzO2 CFB = change from baseline; Source: Trevena data on file |
|
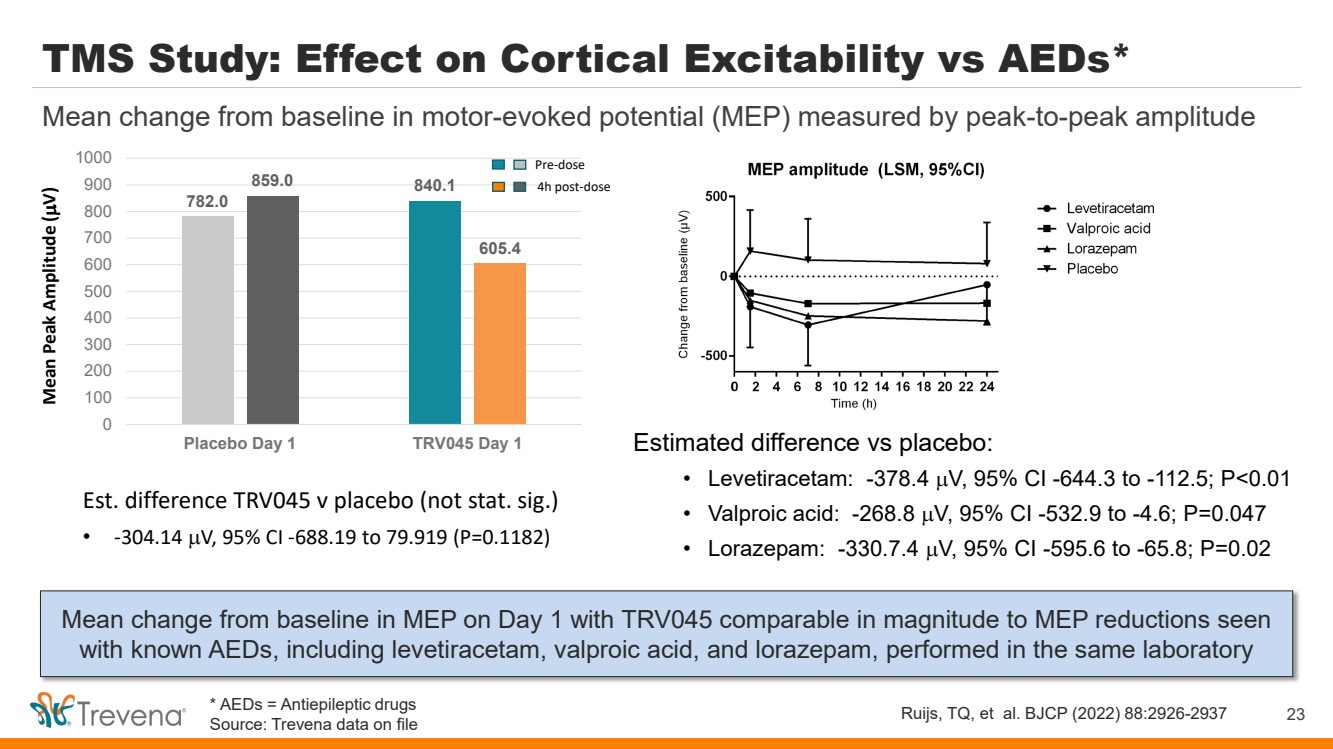
TMS Study: Effect on Cortical Excitability vs AEDs* Mean change from baseline in MEP on Day 1 with TRV045 comparable in magnitude to MEP reductions seen with known AEDs, including levetiracetam, valproic acid, and lorazepam, performed in the same laboratory 23 Mean change from baseline in motor-evoked potential (MEP) measured by peak-to-peak amplitude Ruijs, TQ, et al. BJCP (2022) 88:2926-2937 Estimated difference vs placebo: • Levetiracetam: -378.4 µV, 95% CI -644.3 to -112.5; P<0.01 • Valproic acid: -268.8 µV, 95% CI -532.9 to -4.6; P=0.047 • Lorazepam: -330.7.4 µV, 95% CI -595.6 to -65.8; P=0.02 782.0 840.1 859.0 605.4 0 100 200 300 400 500 600 700 800 900 1000 Placebo Day 1 TRV045 Day 1 Mean Peak Amplitude ( µV) Pre-dose 4h post-dose Est. difference TRV045 v placebo (not stat. sig.) • -304.14 µV, 95% CI -688.19 to 79.919 (P=0.1182) * AEDs = Antiepileptic drugs Source: Trevena data on file |
|
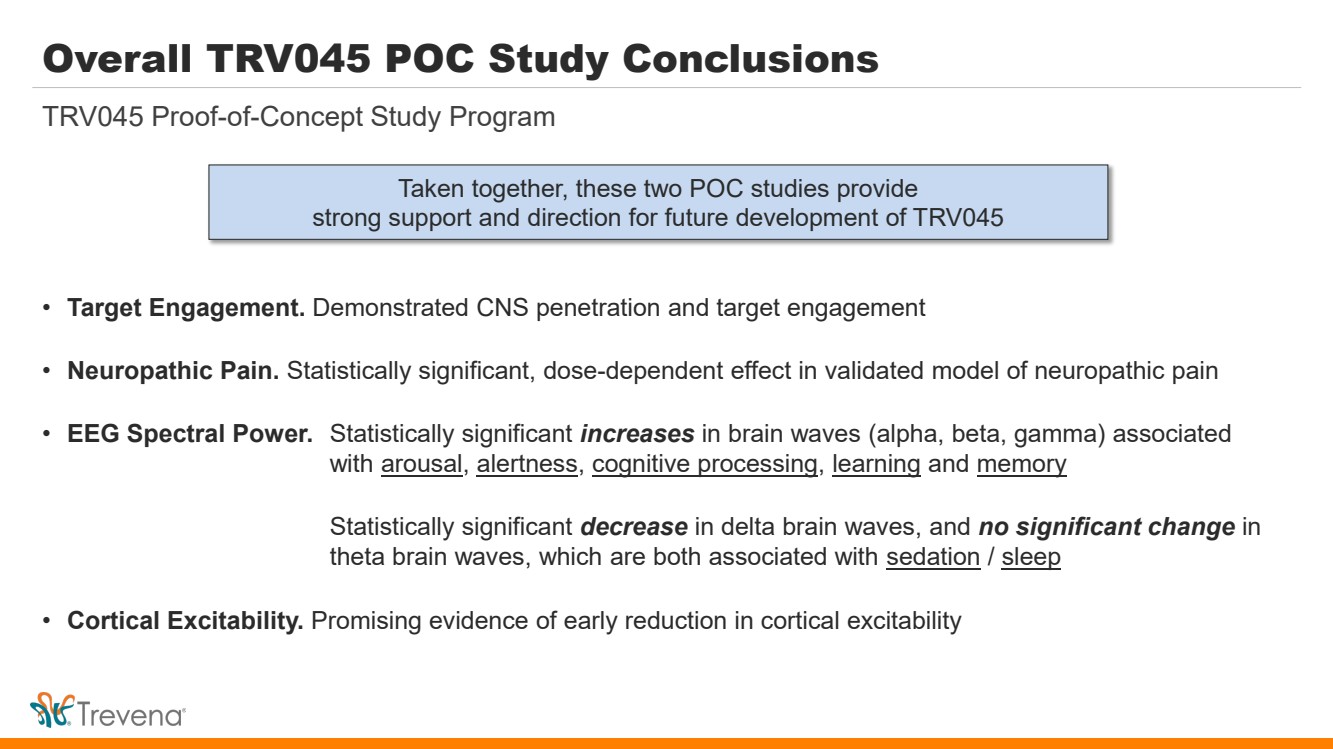
Overall TRV045 POC Study Conclusions • Target Engagement. Demonstrated CNS penetration and target engagement • Neuropathic Pain. Statistically significant, dose-dependent effect in validated model of neuropathic pain • EEG Spectral Power. Statistically significant increases in brain waves (alpha, beta, gamma) associated with arousal, alertness, cognitive processing, learning and memory Statistically significant decrease in delta brain waves, and no significant change in theta brain waves, which are both associated with sedation / sleep • Cortical Excitability. Promising evidence of early reduction in cortical excitability TRV045 Proof-of-Concept Study Program Taken together, these two POC studies provide strong support and direction for future development of TRV045 |
|
TRV734: Maintenance Therapy for Opioid Use Disorder |
|
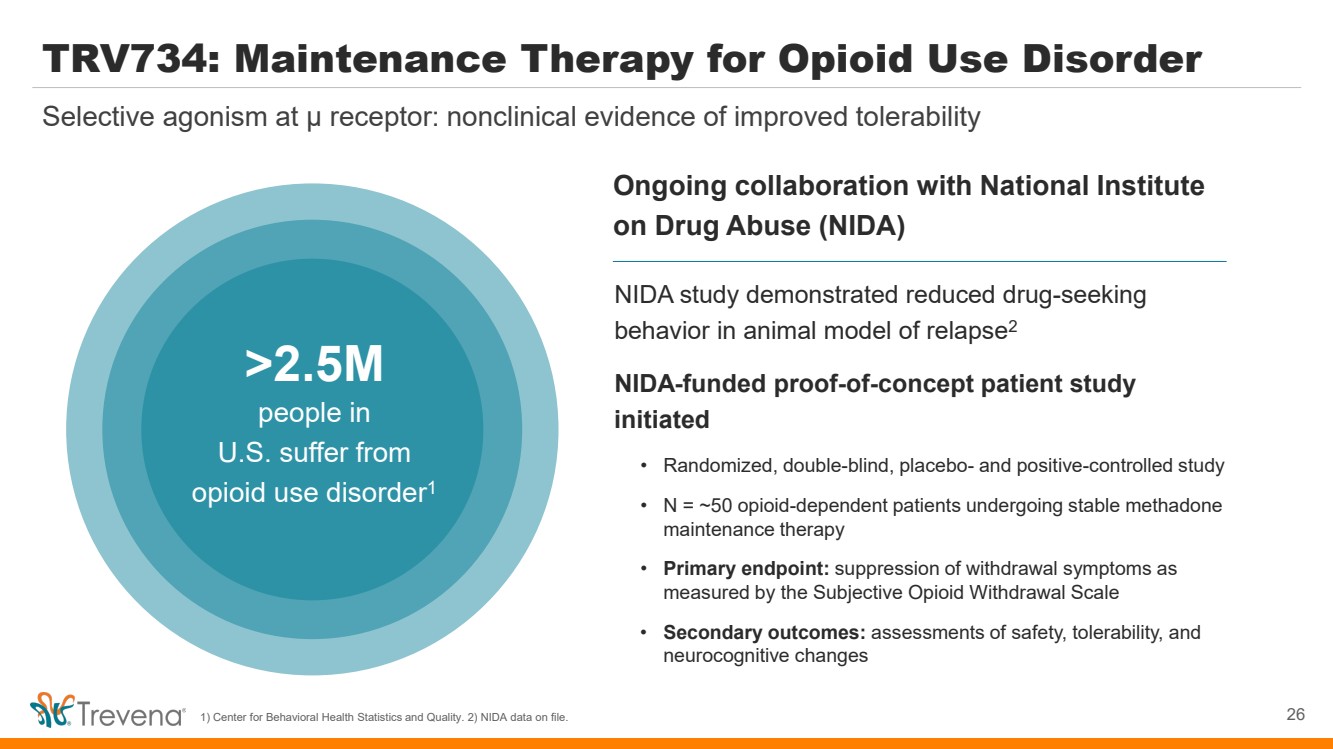
TRV734: Maintenance Therapy for Opioid Use Disorder 26 Selective agonism at µ receptor: nonclinical evidence of improved tolerability 1) Center for Behavioral Health Statistics and Quality. 2) NIDA data on file. Ongoing collaboration with National Institute on Drug Abuse (NIDA) >2.5M people in U.S. suffer from opioid use disorder1 NIDA study demonstrated reduced drug-seeking behavior in animal model of relapse2 NIDA-funded proof-of-concept patient study initiated • Randomized, double-blind, placebo- and positive-controlled study • N = ~50 opioid-dependent patients undergoing stable methadone maintenance therapy • Primary endpoint: suppression of withdrawal symptoms as measured by the Subjective Opioid Withdrawal Scale • Secondary outcomes: assessments of safety, tolerability, and neurocognitive changes |
|
*OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com NCE = New Chemical Entity; PoC = Proof of concept Trevena: Innovative CNS Company 27 IV OLINVYK: Differentiated profile TRV045: Selective S1PR modulator Novel CNS pipeline TRV045: Compelling PoC Data Financial position NCE approved for the management of acute pain in adults* Significant cost savings / differentiation shown in ‘real world’ post-approval studies S1PR: Validated target for blockbusters (fingolimod / siponimod / ozanimod / ponesimod) TRV045: Unique profile ( with potential for no lymphopenia) for new indications New mechanisms for acute / neuropathic pain, epilepsy, acute migraine, opioid use disorder NCEs targeting significant unmet needs Statistically significant, dose-dependent effect in validated model of neuropathic pain Statistically significant EEG changes and evidence of early reduction in cortical excitability $28.1M cash / equivalents / marketable securities @ 2Q 23 $15M non-dilutive tranche received 3Q 23 (ex-US royalty based financing) |
|
28 IMPORTANT SAFETY INFORMATION |
|
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CENTRAL NERVOUS SYSTEM (CNS) DEPRESSANTS Addiction, Abuse, and Misuse OLINVYK exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk before prescribing OLINVYK, and monitor all patients regularly for the development of behaviors or conditions. Life-Threatening Respiratory Depression Serious, life-threatening, or fatal respiratory depression may occur with use of OLINVYK. Monitor for respiratory depression, especially during initiation of OLINVYK or following a dose increase. Neonatal Opioid Withdrawal Syndrome Prolonged use of OLINVYK during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. Risk From Concomitant Use With Benzodiazepines or Other CNS Depressants Concomitant use of opioids with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation. Limitations of Use Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, reserve OLINVYK for use in patients for whom alternative treatment options [e.g., non-opioid analgesics or opioid combination products]: • Have not been tolerated, or are not expected to be tolerated • Have not provided adequate analgesia, or are not expected to provide adequate analgesia. The cumulative total daily dose should not exceed 27 mg, as total daily doses greater than 27 mg may increase the risk for QTc interval prolongation. CONTRAINDICATIONS OLINVYK is contraindicated in patients with: • Significant respiratory depression • Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment • Known or suspected gastrointestinal obstruction, including paralytic ileus • Known hypersensitivity to oliceridine (e.g., anaphylaxis) WARNINGS AND PRECAUTIONS • OLINVYK contains oliceridine, a Schedule II controlled substance, that exposes users to the risks of addiction, abuse, and misuse. Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed OLINVYK. Assess risk, counsel, and monitor all patients receiving opioids. • Serious, life-threatening respiratory depression has been reported with the use of opioids, even when used as recommended, especially in patients with chronic pulmonary disease, or in elderly, cachectic and debilitated patients. The risk is greatest during initiation of OLINVYK therapy, following a dose increase, or when used with other drugs that depress respiration. Proper dosing of OLINVYK is essential, especially when converting patients from another opioid product to avoid overdose. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status. • Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia with risk increasing in a dose-dependent fashion. In patients who present with CSA, consider decreasing the dose of opioid using best practices for opioid taper. INDICATIONS AND USAGE OLINVYK is a new chemical entity indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. 29 |
|
WARNINGS AND PRECAUTIONS • Prolonged use of opioids during pregnancy can result in withdrawal in the neonate that may be life-threatening. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using OLINVYK for a prolonged period of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. • Profound sedation, respiratory depression, coma, and death may result from the concomitant use of OLINVYK with benzodiazepines or other CNS depressants (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, or alcohol). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate, prescribe the lowest effective dose, and minimize the duration. • OLINVYK was shown to have mild QTc interval prolongation in thorough QT studies where patients were dosed up to 27 mg. Total cumulative daily doses exceeding 27 mg per day were not studied and may increase the risk for QTc interval prolongation. Therefore, the cumulative total daily dose of OLINVYK should not exceed 27 mg. • Increased plasma concentrations of OLINVYK may occur in patients with decreased Cytochrome P450 (CYP) 2D6 function or normal metabolizers taking moderate or strong CYP2D6 inhibitors; also in patients taking a moderate or strong CYP3A4 inhibitor, in patients with decreased CYP2D6 function who are also receiving a moderate or strong CYP3A4 inhibitor, or with discontinuation of a CYP3A4 inducer. These patients may require less frequent dosing and should be closely monitored for respiratory depression and sedation at frequent intervals. Concomitant use of OLINVYK with CYP3A4 inducers or discontinuation of a moderate or strong CYP3A4 inhibitor can lower the expected concentration, which may decrease efficacy, and may require supplemental doses. • Cases of adrenal insufficiency have been reported with opioid use (usually greater than one month). Presentation and symptoms may be nonspecific and include nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If confirmed, treat with physiologic replacement doses of corticosteroids and wean patient from the opioid. • OLINVYK may cause severe hypotension, including orthostatic hypotension and syncope in ambulatory patients. • There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension. In patients with circulatory shock, avoid the use of OLINVYK as it may cause vasodilation that can further reduce cardiac output and blood pressure. • Avoid the use of OLINVYK in patients with impaired consciousness or coma. OLINVYK should be used with caution in patients who may be susceptible to the intracranial effects of CO2 retention, such as those with evidence of increased intracranial pressure or brain tumors, as a reduction in respiratory drive and the resultant CO2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy. • As with all opioids, OLINVYK may cause spasm of the sphincter of Oddi, and may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms. • There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension. In patients with circulatory shock, avoid the use of OLINVYK as it may cause vasodilation that can further reduce cardiac output and blood pressure. • Avoid the use of OLINVYK in patients with impaired consciousness or coma. OLINVYK should be used with caution in patients who may be susceptible to the intracranial effects of CO2 retention, such as those with evidence of increased intracranial pressure or brain tumors, as a reduction in respiratory drive and the resultant CO2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy. • As with all opioids, OLINVYK may cause spasm of the sphincter of Oddi, and may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms. • OLINVYK may increase the frequency of seizures in patients with seizure disorders and may increase the risk of seizures in vulnerable patients. Monitor patients with a history of seizure disorders for worsened seizure control. • Do not abruptly discontinue OLINVYK in a patient physically dependent on opioids. Gradually taper the dosage to avoid a withdrawal syndrome and return of pain. Avoid the use of mixed agonist/antagonist (e.g., pentazocine, nalbuphine, and butorphanol) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving OLINVYK, as they may reduce the analgesic effect and/or precipitate withdrawal symptoms. • OLINVYK may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. • Although self-administration of opioids by patient-controlled analgesia (PCA) may allow each patient to individually titrate to an acceptable level of analgesia, PCA administration has resulted in adverse outcomes and episodes of respiratory depression. Health care providers and family members monitoring patients receiving PCA analgesia should be instructed in the need for appropriate monitoring for excessive sedation, respiratory depression, or other adverse effects of opioid medications. ADVERSE REACTIONS Adverse reactions are described in greater detail in the Prescribing Information. The most common (incidence ≥10%) adverse reactions in Phase 3 controlled clinical trials were nausea, vomiting, dizziness, headache, constipation, pruritus, and hypoxia. PLEASE see www.OLNVYK.com for full prescribing information including BOXED warning and important safety information 30 |